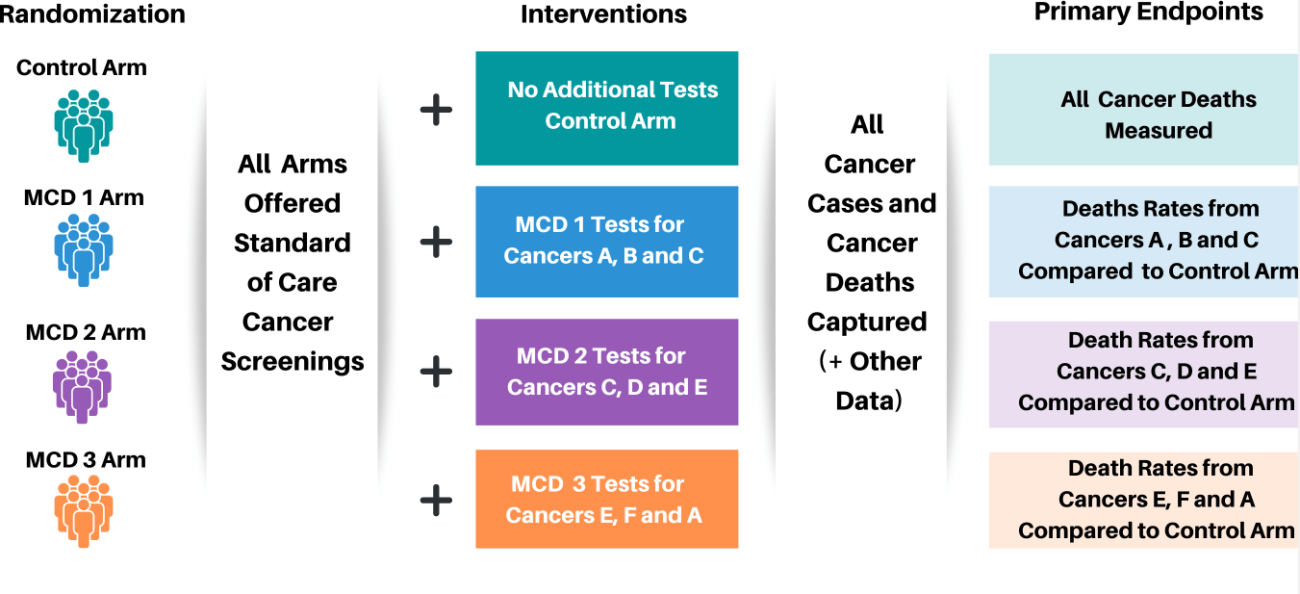
The preliminary design for a large-scale trial will include a control arm that receives no additional tests, an arm that receives one multi-cancer detection test (MCD) that will test for cancers a, b, and c (called MCD 1 arm); a second arm that receives a second MCD test that test for cancers c, d, and e (called MCD 2 arm); and a third arm that receives a third MCD test that test for cancers e, f, and a (called MCD 3 arm).
All cancer cases and cancer deaths will be captured plus other data for all arms of this study.
The primary endpoints for the control arm are all cancer deaths measured.
The primary endpoints for the MCD 1 arm are death rates from cancers a, b, and c compared to the control arm.
The primary endpoints for MCD 2 arm are death rates from cancers c, d, and e compared to the control arm.
The primary endpoints for the MCD 3 arm are death rates from cancers e, f, and a compared to the control arm.