Cancer prevention and early detection research has been thriving at the National Cancer Institute for more than 45 years. Since 1971, the science of cancer prevention has been infused by the guiding principles of reducing cancer risk and detecting cancer early. Whether the focus of cancer prevention research was on developing agents, biomarkers, clinical trials, screening approaches, lifestyle interventions, or measurement validations, the decades of dedicated effort have continued to advance knowledge and help people live longer, healthier lives.
Select the dots or scroll down to read about DCP's important milestones:
1970
1980
1990
2000
2010
2020
2030

1971 December: National Cancer Act Signed
President Richard M. Nixon signs the National Cancer Act, which authorizes the NCI Director to coordinate all activities of the National Cancer Program, to establish national cancer research centers, and to establish national cancer control programs.
1974 NCI Awards Cancer Control Grants for Ovarian Screenings
Cancer control grants are awarded to state health departments to increase ovarian cancer screenings for low-income women.
1977 Senate Select Committee on Nutrition Produces Report on US Dietary Goals
Lead by Senator George McGovern, the Senate Select Committee on Nutrition produces a report on “The Dietary Goals for the United States.” This publication recommends a low-fat, high-fiber diet to lower the risk of diseases such as diabetes and cancer. The report stimulates interest and increased research in the area of nutrition.
1978 Community Hospital Oncology Program Originates
NCI begins to organize the Community Hospital Oncology Program (CHOP), a predecessor of the Community Clinical Oncology Program (CCOP).
1979 Public Health Service Publication Provides Diet Guidelines
The Surgeon General publishes “Healthy People: Health Promotion and Disease Prevention,” which provides nutrition advice. This is the first public health service publication to provide diet guidelines.

1979 October: NCI Diet Recommendations Announced
NCI announces that a balanced, low-fat diet can reduce the risk of roughly 30% of cancers. Diet recommendations include low alcohol intake and increased amounts of fiber.
1981 October: NCI Approves Concept for Community Clinical Oncology Program (CCOP)
The concept for a community-based program is approved by NCI. The program is aimed at providing cancer care and research to underserved areas and creating a conduit for technology transfer.
1982 Chemoprevention Research Program Established
The chemoprevention research program is established to locate cancer-reducing micronutrients or synthetic compounds.
1982 NCI Provides Grants for Cancer Prevention Research under Small Business Innovation Law
Congress authorizes the Small Business Innovation Research (SBIR) Program under the Small Business Innovation Development Act (P.L. 97-219) to stimulate private sector contributions to federal research and development needs. NCI uses this program to provide grants for cancer prevention research.
1982 Smoking, Tobacco, and Cancer Intervention Research Program Launched
The Smoking, Tobacco, and Cancer Program (STCP) intervention research program is launched to test specific intervention conduits such as the media, schools, and health care providers.
1983 New Prevention Research Program Begins
NCI begins a new program to address prevention research in diet, chemoprevention, early detection, and identification of high-risk occupations.
1983 July: NCI Funds the New Community Clinical Oncology Program (CCOP)
The CCOP, 62 clinical oncology centers throughout the nation that are responsible for enrolling patients in NCI trials, is funded. These centers conduct cancer treatment, prevention, and control clinical trials in an effort to develop clinical prevention and symptom management.
1983 December: NCI Adds New Division Devoted to Prevention and Control
The NCI creates the Division of Cancer Prevention and Control (DCPC).
1984 Division of Cancer Prevention and Control (DCPC) Starts Programs, Trials, and Studies
The DCPC establishes the Cancer Control Science Program to publicize cancer control knowledge. Clinical trials in chemoprevention and diet and nutrition begin, and the DCPC adds preclinical efficacy and toxicology studies.
1984 May: NCI Discusses Dietary Health Claims with Company
NCI meets with the Kellogg Company to discuss health claims in a publicity campaign for All-Bran cereal that promotes the cancer prevention benefits of high-fiber, low-fat foods.
1985 May: Coordination of Linxian China Dysplasia Trial Starts
The Linxian China Dysplasia Trial begins with coordination between the Cancer Institute of the Chinese Academy of Medical Sciences and the NCI. People with severe esophageal dysplasia begin taking either vitamin and mineral supplements or placebos in order to test the etiologic and preventative role of vitamins and minerals in the late stages of esophageal carcinogenesis.
1985 Alpha-Tocopherol, Beta Carotene (ATBC) Cancer Prevention Study Starts
The purpose of the ATBC study was to determine whether certain vitamin supplements would prevent lung cancer and other cancers in a group of 29,133 male smokers in Finland. The 50- to 69-year-old participants took a pill daily for five to eight years that contained either alpha-tocopherol (a form of vitamin E), beta-carotene (a precursor of vitamin A), both, or a placebo (inactive pill that looked like the vitamin).
1986 Community Clinical Oncology Program Expands to Large Prevention and Control Trials
CCOP expands its research portfolio to include large-scale cancer prevention and control trials.
1987 NCI Develops Working Guidelines for Cervical and Breast Cancer Screenings
Along with other organizations, the NCI develops Working Guidelines for cervical and breast cancer screenings, including regular mammograms by age 40 and annual mammograms after age 50.
1987 Cancer Prevention Fellowship Program (CPFP) Begins
The CPFP is instituted to provide clinicians and scientists with a strong foundation to train in the field of cancer prevention and control. The fellowship offers training toward an M.P.H. degree at an accredited university during the first year, followed by mentored research with investigators at the NCI, and opportunities for cutting-edge research in the basic, quantitative, social and behavioral sciences, and clinical cancer prevention.
1988 Law Mandates Medicare Coverage of Mammography Screenings
President Reagan signs P.L. 100-360, The Medicare Catastrophic Coverage Act of 1988. It mandates that as of January 1, 1990, mammography provisions include biennial screening for women aged 65 and older and annual screenings for women aged 50 to 64. Women who are between the ages of 40 and 49 should have screenings available every other year, and by the time a woman is 40 she should have at least one mammogram.
1989 January: NCI Approves the Minority-Based Community Clinical Oncology Program (MB-CCOP)
NCI approves the MB-CCOP, which is aimed at providing minorities and people in underserved areas greater access to preventative trials and cancer treatment.

1991 American Stop Smoking Intervention Study (ASSIST) Starts
Working in conjunction, the American Cancer Society and the Division of Cancer Prevention and Control (DCPC) announce the start of ASSIST. The study sets the goal of having a smoke-free society by 2000.
1991 National 5-A-Day Public Health Message Supported
In conjunction with the Produce for Better Health Foundation, the Division of Cancer Prevention and Control (DCPC) begins supporting the National 5-A-Day message, which encourages the American public to eat at least five servings of fruits and vegetables a day.
1991 Chemoprevention, Intervention Trials Underway
The Division of Cancer Prevention and Control (DCPC) is responsible for 27 intervention clinical trials, including synthetic retinoids and natural chemoprevention substances such as vitamins A, C, E, B12, folate, and beta-carotene.

1992 April: Breast Cancer Prevention Trial (BCPT) Begins Enrollment
The BCPT begins enrolling women to test the chemopreventive drug tamoxifen. A total of 13,388 women age 35 and older who are at increased risk of breast cancer are accepted into the trial.
1993 October: NCI Convenes International Workshop on Breast Cancer Screening
An NCI-convened international workshop on breast cancer screening reviews published and unpublished data from eight randomized controlled clinical trials, including the HIP trial that was launched in 1963, and concludes that screening with mammography reduces breast cancer mortality among women ages 50-69.

1993 October: Enrollment for the Prostate Cancer Prevention Trial Begins
PCPT starts enrolling participants. The intention of the 18,882-man trial is to determine whether or not the drug finasteride can prevent prostate cancer in men. The drug is approved for the treatment of benign prostatic hyperplasia, but it is believed that it also reduces levels of the male hormone that is involved in prostate cancer development.

1993 December: NCI Drops Mammography Guidelines
The NCI drops its previous mammography guidelines by no longer advising that women under 50 should have annual mammograms.
1993 Trial Results Show Fecal Occult Blood Test (FOBT) Screening Reduces Colorectal Cancer Mortality
Results from an NCI-supported, randomized controlled clinical trial show that annual screening with guaiac FOBT can help reduce colorectal cancer mortality by about 33%.
1993 CCOP Network Helps with Colorectal Adenoma Prevention Study (CAPS)
The CCOP network assists the Cancer and Leukemia Group B in the CAPS. This study evaluates the effectiveness of aspirin in reducing early-stage colorectal tumors.
1993 Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Begins Enrollment
Participant enrollment begins for the PLCO trial, a large population-based randomized clinical trial evaluating cancer screening by chest x- rays, flexible sigmoidoscopy, transvaginal ultrasound, blood tests, and digital rectal exams in more than 150,000 men and women ages 55 to 74.

1994 Trial Finds Beta-Carotene Increases Lung Cancer in Finnish Males
The Alpha-Tocopherol (vitamin E)/ Beta-Carotene Prevention Study (ATBC) findings indicate that beta-carotene in fact increases lung cancer incidence in Finnish males.

1996 ASCUS/LSIL Triage Study (ALTS) Begins Enrollment
ALTS starts enrolling participants. This study concerns treatment of mild abnormalities found on the cervix during Pap tests. Women in the trial might have: immediate colposcopy, no treatment until the screenings indicate a high-grade lesion, or human papillomavirus (HPV) testing.
1997 NCI Establishes Separate Divisions for Prevention and Control/Population Sciences
The NCI reorganizes and divides the Division of Cancer Prevention and Control (DCPC) into the Division of Cancer Prevention (DCP) and the Division of Cancer Control and Population Sciences (DCCPS). The DCP focuses more exclusively on cancer prevention, while the DCCPS explores population genetics, epidemiology, behavior, society, and welfare of cancer survivors.

1998 Breast Cancer Prevention Trial (BCPT) Shows Tamoxifen Reduces Breast Cancer Incidence
Results from the BCPT show that the drug tamoxifen reduces the incidence of breast cancer by 49% among women who are at increased risk of the disease. The Food and Drug Administration subsequently approved tamoxifen for the prevention of breast cancer in high-risk women.
1999 Early Detection Research Network (EDRN) is Founded
The EDRN is established to coordinate development, evaluation, and application of biomarkers of cancer and cancer risk in a network of institutions. EDRN includes biomarker developmental labs, clinical and epidemiologic centers, biomarker validation labs, and a data management and coordinating center.
1999 Rapid Access to Preventive Intervention Development (RAPID) Program Begins
DCP begins to develop the RAPID program, which provides contract support to investigators who need help to advance laboratory and clinical testing on various chemopreventive agents and molecules.
1999 Adenoma Prevention with Celecoxib (APC) Trial Starts Enrollment
Enrollment for the APC clinical trial begins, assigning more than 2,000 participants 200 mg of celecoxib (Celebrex®) twice a day. The drug is expected to block cyclooxygenase (COX) enzymes, which are turned on by precancerous and cancerous tissues. The trial is part of a larger search for effective non-steroidal anti-inflammatory drugs acting as cancer-preventing mechanisms.

1999 Study of Tamoxifen and Raloxifene (STAR) Commences
The STAR clinical trial begins. Its goal is to compare the effectiveness of both drugs in reducing breast cancer incidence and to compare adverse side effects. Over 22,000 women spanning 200 clinics will take one of the two drugs for 5 years. Enrollment is completed in November 2004.

2000 April: Polyp Prevention Trial Finds Low-Fat, High Fiber Diet Does Not Influence Risk of Recurrence of Colorectal Adenomas
The Polyp Prevention Trial tested the hypothesis that dietary intervention can inhibit the development of recurrent colorectal adenomas, the precursors of most large-bowel cancers. The study results found that adopting a diet that is low in fat and high in fiber, fruits, and vegetables does not influence the risk of recurrence of colorectal adenomas. The primary objective was to determine whether a low fat, high fiber, high vegetable and fruit eating plan would decrease the recurrence of adenomatous polyps of the large bowel. Secondary objectives were to: evaluate effectiveness of the intervention program with respect to participant achievement of dietary goals; examine dietary changes and associated biochemical markers in blood; and assess impact on quality of life.

2001 July: Selenium and Vitamin E Cancer Prevention Trial (SELECT) Begins Recruiting Men
The SELECT starts recruiting men over the age of 55 to test the effectiveness of these two dietary supplements as prostate cancer prevention agents.

2002 National Lung Screening Trial (NLST) Begins
The NLST trial is launched. It is aimed at comparing the effectiveness of two early lung cancer detection tests, single-view chest x-ray and spiral-computed tomography.

2002 May: Results from the Colorectal Adenoma Prevention Study (CAPS) Released
Results from the CAPS clinical trial indicate that daily use of aspirin can reduce the development of colorectal tumors by 35% in patients with a pre-existing history of polyps.

2002 Initial Results Released from the ASCUS/LSIL Triage Study (ALTS) on HPV Testing
Initial results from the ALTS indicate that HPV testing can determine whether atypical squamous cells of undetermined significance (ASCUS) will progress into cervical cancer. HPV testing is found not useful for women with low-grade lesions due to the high incidence of HPV in women with these specific abnormalities.

2003 June: Results from the Prostate Cancer Prevention Trial (PCPT) Released
PCPT results show that the drug finasteride, which reduces the production of male hormones in the body, lowers a man's risk of prostate cancer by about 25%, demonstrating that prostate cancer, like breast cancer, can be prevented.

2004 August: “Decades of Progress 1983 to 2003” Published
The first 20 years of the NCI Community Clinical Oncology Program (CCOP), the precursor to the NCI Community Oncology Research Program (NCORP), are documented.
2004 December: Adenoma Prevention with Celecoxib (APC) Trial Suspended
The APC clinical trial is suspended based on an increased incidence of major cardiovascular events in participants taking celecoxib (Celebrex®). Other NCI trials continue with a revision to consent forms, and research into the benefits of celecoxib continues. Despite suspension of the trial and obvious negative side effects, the DCP observes 33% to 45% fewer adenomas in those within the APC trial, indicating further research possibilities such as anti-inflammatory pathways.
2005 November: Results of the Breast Cancer Prevention Trial (BCPT) Updated
Updated BCPT results show a continued reduction of invasive breast cancer incidence as well as a decrease in some negative side effects. These side effects, first noted following the 1998 study, include increased risk of stroke, pulmonary embolism, and deep vein thrombosis.

2006 February: Low-Fat Diet May Have Small Impact on Breast Cancer in Women
Results from the Women's Health Initiative (WHI) Dietary Modification (DM) prevention study show that reducing dietary fat and increasing fruits, grains, and vegetables may reduce risk of invasive breast cancer in some women, but has no effect on invasive colorectal cancer. Although more than 19,500 postmenopausal women followed the modified diet, the overall breast cancer risk reduction of 9% was not statistically significant after an average of 8.1 years. Women whose diets were highest in fat before they entered the study, however, were 22% less likely to develop breast cancer than the comparison group.

2006 April: Initial Results of the Study of Tamoxifen and Raloxifene (STAR) Released
Initial results of the STAR clinical trial show that postmenopausal women who are at increased risk of breast cancer can reduce their risk of developing the disease if they take the drug raloxifene, an antiestrogen agent already approved by the Food and Drug Administration for the treatment and prevention of osteoporosis. Mature results from STAR later show that raloxifene is somewhat less effective than tamoxifen in preventing breast cancer but that it also has less toxicity, including a substantially lower risk of endometrial cancer.

2007 January: Low-Fat Diet May Help Prevent Breast Cancer Recurrence
Findings from the NCI-sponsored Women's Intervention Nutrition Study (WINS) was published. Interim results from the first large-scale randomized clinical trial testing an intervention show that women who reduce their consumption of fat after treatment for early-stage breast cancer may also reduce their risk of a breast cancer recurrence.

2007 July: Adopting Diet High in Vegetables, Fruit, Fiber, Low in Fat Did Not Reduce Breast Cancer Events or Mortality for Early Stage Breast Cancer Survivors
The published results of the Women's Healthy Eating and Living (WHEL) randomized trial show that among survivors of early stage breast cancer, adoption of a diet that was very high in vegetables, fruit, and fiber and low in fat did not reduce additional breast cancer events or mortality during a 7.3-year follow-up period.

2008 October: Initial results from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Released
Initial results from the SELECT clinical trial indicate that selenium and vitamin E do not contribute to the prevention of prostate cancer. In fact, test results suggest a slight increase in prostate cancer incidence in subjects taking vitamin E.

2009 March: Prostate Results from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial Released
Results from the PLCO trial show that screening men 55 years of age and older with PSA tests and digital rectal exams was not effective in reducing prostate cancer mortality.
2010 February: Alliance of Glycobiologists for Detection of Cancer Identify Key Antitumor Antibodies
The Alliance of Glycobiologists for Detection of Cancer find that cancer patients produce antibodies that target abnormal proteins with sugar molecules attached made by their tumors, suggesting that antitumor antibodies in the blood may be a source of sensitive biomarkers for cancer detection.
2010 August: Early Detection Research Network (EDRN) Continues as New Grants Awarded
The EDRN continues the biomarker discovery and validation program with the awarding of 32 new grants to fund 20 biomarker development laboratories, eight clinical validation centers, three biomarker reference laboratories, and a data coordinating management and center.

2010 November: Initial Results of the Lung Cancer Screening Trial (NLST) Released
Initial results of the NLST clinical trial show that screening with low-dose helical computerized tomography (CT) reduced lung cancer deaths by about 20% among current and former heavy smokers.

2011 January: “Accomplishments in Cancer Clinical Trials” Published
Highlights of the NCI Community Clinical Oncology Program (CCOP), the precursor to the NCI Community Oncology Research Program (NCORP), are updated in a report.

2011 June: Ovarian Results from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Released
PLCO results show that screening for ovarian cancer with transvaginal ultrasound (TVU) and the CA-125 blood test did not result in fewer deaths from the disease compared with usual care. In addition, false-positive results from the two screening methods often led to unnecessary surgeries and other serious complications.

2011 October: Lung Results from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Released
PLCO clinical trial results show that annual screening for lung cancer using a standard chest x-ray does not reduce the risk of dying from lung cancer when compared with no annual screening.

2012 January: Prostate Data Updated from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial
PLCO trial data show that after 13 years of follow-up, men who underwent annual prostate cancer screening with prostate-specific antigen (PSA) testing and digital rectal examination had a 12% higher incidence of prostate cancer than men in the control group, but had the same rate of death from the disease. No evidence of a mortality benefit from screening was seen in subgroups defined by age, the presence of other illnesses, or pre-trial PSA testing.

2012 May: Colorectal Results from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Released
Results of the PLCO trial confirm that screening people 55 years of age and older for colorectal cancer using flexible sigmoidoscopy reduces colorectal cancer incidence and mortality. In the PLCO, screened individuals had a 21% lower risk of developing colorectal cancer and a 26% lower risk of dying from the disease than the control subjects.

2013 June: NCI Community Oncology Research Program (NCORP) Approved for Start
NCORP is approved by the NCI Board of Scientific Advisors, opening the way for the program to bring state-of-the art cancer prevention, control, treatment and imaging clinical trials, cancer care delivery research, and disparities studies to individuals in their own communities. It replaces, but expands on the success of the Community Clinical Oncology Program (CCOP), including the Minority-Based CCOPs; supersedes, but adds elements of the NCI Community Cancer Centers Program (NCCCP); and creates a network for cancer care delivery research.

2013 August: Prostate Cancer Prevention Trial (PCPT) Findings Updated
Based on follow-up of trial participants for up to 18 years, the PCPT updated findings show that survival of the men on finasteride is equivalent to men who did not take the drug, and the reduction in risk of prostate cancer persists.

2013 December: National Lung Screening Trial (NLST) Researchers Issue Finding on Overdiagnosis
A detailed analysis of the primary NLST findings showed that screening can detect slow-growing tumors that otherwise may not cause clinical symptoms, and can lead to overdiagnosis. The probability was 18.5% that any lung cancer detected by screening with low-dose CT was an overdiagnosis, and 22.5% that a non-small cell lung cancer, the most common form, detected by low-dose CT was an overdiagnosis. Overdiagnosis represents the potential harm of additional cost, anxiety, and morbidity associated with treatment.

2014 February: Selenium and Vitamin E Cancer Prevention Trial (SELECT) Findings Updated
In an update, SELECT researchers report that men who had high levels of selenium at the start of the trial, as assessed by measures of selenium in their toenail clippings, had almost double the chance of developing a high-grade prostate cancer if they took the selenium supplement compared to men with low levels of selenium at the start of the trial. Additionally, men with low selenium levels at the start of the trial had double the chance of developing a high-grade prostate cancer if they took the vitamin E supplement.

2014 May: Prevention of Early Menopause Study (POEMS) Clinical Trial Results Announced
The POEMS clinical trial results show young women with breast cancer were able to better preserve their fertility during cancer treatments by using hormone-blocking drug injections that put them into temporary menopause.

2014 July: Rationale and Design of the Men's Eating and Living (MEAL) Study Published
The MEAL Study begins assessing the effectiveness of a high-vegetable diet intervention for preventing clinical progression in men with early stage localized prostate cancer on active surveillance.

2014 August: NCI Community Oncology Research Program (NCORP) Gets Underway
The NCORP awards 53 new 5-year grants to researchers across the country to conduct multi-site cancer clinical trials and cancer care delivery research studies in their communities.

2015 January: Cancer Prevention and Control Central Institutional Review Board (CIRB) Established
The addition of the Cancer Prevention and Control (CPC) CIRB extends the benefits of centralized IRB review to investigators participating in clinical trials sponsored by the Division of Cancer Prevention. The CPC CIRB’s role is to review studies developed by the DCP-sponsored NCI Community Oncology Research Program (NCORP) and the Consortia for Early Phase Trials Program.
2015 June: NCORP Sites Participate in Enrolling Patients in the NCI-MATCH (Molecular Analysis for Therapy Choice) Precision Medicine Trial
The trial seeks to determine whether targeted therapies for people whose tumors have specific gene mutations will be effective regardless of their cancer type.

2015 July: DCP Leads a Key Initiative in the National Institutes of Health (NIH) Glycoscience Common Fund Program
The program supports the development of accessible and affordable new tools and technologies for studying the role complex carbohydrates in health and disease.
2015 August: Consortium on Imaging and Biomarkers is Created with Grants to Eight Principal Investigators
The consortium focuses on combining imaging methods with biomarkers to improve the accuracy of screening, early cancer detection, and diagnosis of early stage cancers. Imaging information is obtained from various means, such as computed tomography (CT), positron emission tomography (PET), magnetic resonance (MR) imaging, and optical-based imaging for real-time visualization of lesions.
2015 October: NCI Awards Grants to Create the Consortium for Molecular Characterization of Screen-Detected Lesions
Supported by the Division of Cancer Prevention and the Division of Cancer Biology, the consortium has seven molecular characterization laboratories and a coordinating center, and focuses on the critical areas of characterizing molecular and cellular features of screen-detected pre-cancers and early cancers, and the tumor microenvironment.

2016 February: The White House Announces $1 billion in Investments in the National Cancer Moonshot initiative
Prevention, including cancer vaccine development and early cancer detection, are two of the five opportunity areas.

2016 May: Largest Ever US Study to Research Causes and Genetics of Blood Diseases Launched
NCI and the National Heart, Lung, and Blood Institute fund the study of myelodysplastic syndromes ( MDS) and unexplained, persistent low red blood cell count (anemia) to build a national research resource. Patients are being recruited through NCI’s Community Oncology Research Program (NCORP) and National Clinical Trials Network.
2016 May: Ovarian Cancer Study Tests Lead Time of Potential Biomarkers
Teams of scientists around the world, such as the Early Detection Research Network, are working to develop ways to detect early signs of ovarian cancer in blood.

2016 June: Data from the Interactive Diet and Activity Tracking in AARP Study (IDATA) Are Made Available to Qualified Investigators
Investigators looking into the relationship between physical activity, diet, and disease can request access to IDATA Study data via the
2016 July: Olanzapine Helps Prevent Nausea and Vomiting Caused by Chemotherapy
Findings from a large phase III clinical trial show a drug currently used to treat several psychiatric conditions can help prevent nausea and vomiting in patients receiving chemotherapy.

2016 October: Study Confirms Benefits of Early Palliative Care for Advanced Cancer
Patients who received palliative care along with standard treatment for advanced cancer reported having a better quality of life and mood than patients who did not receive early palliative care.
2016 December: Think Tank Emphasizes Identifying and Creating the Next Generation of Community-Based Cancer Prevention Studies
Cancer prevention research experts and stakeholders discuss the state of cancer prevention research, identify key prevention research priorities for NCI, and identify studies that could be conducted within the NCI Community Oncology Research Program (NCORP).
2017 May: NCI’s Cancer Prevention Fellowship Program (CPFP) Celebrates 30 Years
As the NCI Cancer Prevention Fellowship Program (CPFP) celebrates its 30th anniversary, the successful cycle continues with the call for applications for the next class of fellows to start in 2018.

2017 July: NCI Joins Leading Groups on Disparities Statement
NCI joins the American Society of Clinical Oncology, American Association for Cancer Research and the American Cancer Society in releasing a statement in the Journal of Clinical Oncology to guide cancer health disparities research.
2017 October: TMIST Trial Aims to Provide Clarity on Breast Cancer Screening Approaches
A large nationwide clinical trial, the Tomosynthesis Mammographic Imaging Screening Trial (TMIST), is launched to try to answer some important questions about the technologies used to screen for breast cancer.
2017 October: Pre-Cancer Atlas and Other Human Tumor Atlas Network Funding Opportunity Announcements Released
Three funding opportunity announcements about the Pre-Cancer Atlas, associated with the Beau Biden Cancer MoonshotSM Initiative, are released with a goal to accelerate cancer research.
2017 November: Experimental Ovarian Cancer Vaccine Shows Promise in Mice
In mouse studies, an experimental vaccine strategy has shown promise for preventing ovarian cancer. The vaccine targets a protein that is present at elevated levels in approximately 90% of human ovarian epithelial cancers, the most common type of ovarian cancer.

2018 May: Finasteride is Found to be Safe in Long-Term Study Results
Twenty-five years after it opened for enrollment, the landmark Prostate Cancer Prevention Trial (PCPT) showed that finasteride, a common hormone-blocking drug, reduces men's risk of getting prostate cancer without increasing their risk of dying from the disease.

2018 July: NCI and VA Collaborate to Boost Veterans’ Access to Cancer Clinical Trials
The NCI and VA Interagency Group to Accelerate Trials Enrollment, or NAVIGATE, launched at 12 VA facilities to enhance the ability of veterans with cancer to participate in trials of novel cancer treatments carried out through NCI’s National Clinical Trials Network (NCTN) and the NCI Community Oncology Research Program (NCORP).

2018 September: Daily Low-dose of Aspirin Found to Have No Effect on Healthy Life Span in Older People
Initial findings from the ASPirin in Reducing Events in the Elderly (ASPREE) trial showed that daily low-dose aspirin did not prolong healthy, independent living (life free of dementia or persistent physical disability) in healthy older adults without previous cardiovascular events.

2018 October: Hormone Injections Reduce Early Menopause, POEMS Trial Results Show
The final, 5-year results of the Prevention of Early Menopause Study (POEMS) show that giving some younger women with early-stage breast cancer a hormone-suppressing drug in addition to chemotherapy helps to preserve ovarian function and improves their ability to get pregnant after treatment. Women who get injections of goserelin along with standard breast cancer chemotherapy were more likely to become pregnant without developing negative side effects or shortening their lives, the NCI-sponsored study found.

2018 November: Vitamin D Supplements Don’t Reduce Cancer Incidence
In the largest-ever randomized clinical trial testing vitamin D for cancer prevention, the supplement did not reduce the risk of developing cancer. The main goal of the Vitamin D and Omega-3 Trial (VITAL) was to see if there’s benefit to getting above the recommended dietary allowance, more than what is considered necessary for bone health.

2018 December: Testing a Topical Drug for Breast Cancer Prevention
To explore alternatives to oral tamoxifen, which can help prevent breast cancer in women at an increased risk of the disease, that might have fewer side effects, researchers are testing a topical form of the drug in two clinical trials. These randomized placebo-controlled studies are evaluating a gel formulation of tamoxifen called 4-hydroxytamoxifen (4-OHT) that women apply directly to the breasts. (Read the NCI Blog)

2019 January: Final Verdict on Finasteride Published
Long-term data published on finasteride, a generic hormone-blocking drug that reduced the risk of prostate cancer by 25% in the landmark Prostate Cancer Prevention Trial (PCPT), showed that reduction in prostate cancer risk has continued since results were previously published and that finasteride has the lasting effect of reducing prostate cancer risk. Fewer than 100 men on the trial had died from the disease.

2019 March: NCORP Tissue Procurement Protocol Opens at 12 Community Sites
At 12 NCI Community Oncology Research Program (NCORP) sites a new kind of study was opened for patients with advanced malignancies being treated with molecularly targeted therapies. The NCORP Tissue Procurement Protocol is not a treatment trial, but a study to assess if researchers can obtain for later analysis tissue and blood samples at baseline and upon progression in patients with advanced cancer being treated with molecularly targeted therapies.

2019 April: Researchers take First Steps Toward Developing a Vaccine to Prevent Cancer in People with Lynch Syndrome
DCP’s PREVENT Cancer Preclinical Drug Development Program, which focuses on unmet needs in cancer prevention, recognized the potential of a vaccine to prevent cancer in individuals with Lynch syndrome, an inherited condition that elevates a person’s risk of colorectal, endometrial, and other types of cancer. Scientists reported results showing that the vaccine prevented the growth of colorectal tumors in a mouse model of Lynch syndrome and prolonged the mice’s survival compared with unvaccinated mice.

2019 August: NCI Community Oncology Research Program (NCORP) Expands to More State
NCI awarded 53 grants to researchers in the NCI Community Oncology Research Program (NCORP) to conduct multi-site cancer clinical trials and cancer care delivery studies in their communities. The awards went to 32 Community Sites and 14 Minority/Underserved Community Sites, who have assembled more than 1,000 affiliates across the country to carry out research. The network covers 44 states, the District of Columbia, and the territories of Puerto Rico and Guam, the largest geographic coverage in the program’s history.

2019 September: ULACNet Aims to Optimize Clinical Interventions for Prevention of HPV-related Cancers in People Living with HIV
The United States-Latin American-Caribbean Clinical Trials Network (ULACNet) is developing evidence to improve and optimize approaches for prevention of human papillomavirus (HPV)-related cancers in people living with human immunodeficiency virus (HIV) infection. The research collaboration brings together institutions in the United States and counterparts in low- and middle-income countries in the Latin American and Caribbean region.

2020 January: Behavioral Interventions Do Not Reduce Prostate Cancer Progression Among Men with Early Stage Disease
Among men with early-stage prostate cancer managed with active surveillance, a behavioral intervention that increased vegetable consumption did not significantly reduce the risk of prostate cancer progression, according to the published results of the Men’s Eating and Living (MEAL) study. The findings do not support using this intervention to decrease prostate cancer progression in this population.
2020 February: New Technology Gives Patients Access to a 5-Minute, Office-Based Test to Identify Risk for Esophageal Cancer
A new technology coupled with a new biomarker test in clinical trials give patients timely access to a quick, accurate and less invasive way to identify risk for one type of esophageal cancer. EsoCheck™ and EsoGuard™ are the device and test created for the detection of Barrett's esophagus, the benign and treatable precursor condition to esophageal adenocarcinomas. The technology was made more widely available with a special Food and Drug Administration designation.
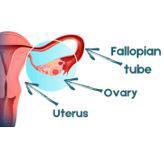
2020 June: SOROCk Trial Seeks to Compare Surgical Approaches in Women at Risk for Ovarian Cancer
A large nationwide clinical trial, Salpingo-Oophorectomy to Reduce the risk of Ovarian Cancer (SOROCk), launches. The study is examining whether removal of the fallopian tubes prevents ovarian cancer in high-risk, premenopausal women as effectively as the standard of care, removal of the fallopian tubes and ovaries.

2020 July: Study with Common Pain Drug Shows Change in Immune Biomarkers, Highlights Pathway for Prevention of Colorectal Cancer in People with Lynch Syndrome
A phase I clinical trial finds that naproxen, an over-the-counter anti-inflammatory drug, activates immune system cells in the innermost layer of the colon in patients with Lynch syndrome. The results suggest that naproxen may be used to prevent colorectal cancer in high-risk individuals. Eduardo Vilar-Sanchez, M.D., Ph.D., is the principal investigator of the study.

2020 August: Regular Aspirin Use May Increase Older People’s Risk of Dying from Cancer
New findings from a randomized clinical trial, ASPirin in Reducing Events in the Elderly (ASPREE), show that older adults who took low-dose aspirin daily had a greater likelihood of being diagnosed with an advanced cancer and of dying from cancer. The results contrast with earlier studies, which observed benefits but mainly involved younger individuals.

2021 January: Second Report Suggests No Benefit to Starting Aspirin Therapy for Cancer Prevention for Adults Aged 70 Years and Older
A pooled analysis of two large U.S. cohorts of health professionals found that starting regular aspirin at 70 years of age or older was not associated with a lower risk of developing colorectal cancer. In contrast, individuals who took daily aspirin for 5 or more years before age 70 years cut their colorectal cancer risk by as much as 20%. Andrew Chan, M.D., M.P.H., is the principal investigator of the study.

2021 December: Monograph Details Opportunities and Challenges in Cancer Research Involving Cannabis
A series of papers is published, providing an overview of presentations and discussions from the NCI Cannabis, Cannabinoids, and Cancer Research Symposium. The meeting identified promising areas of future study, current barriers to conducting cancer cannabis research, and strategies to overcome difficulties.
