Date Posted, by DCP Staff
EsoCheck™ and EsoGuard™
NCI Annual Plan & Budget Proposal for Fiscal Year 2020 (PDF, 17.4 MB)
A new technology coupled with a new biomarker test now in clinical trials are giving patients timely access to a quick, accurate and less invasive way to identify risk for one type of esophageal cancer.
EsoCheck™ and EsoGuard™ are the device and test created for the detection of Barrett's esophagus, the benign and treatable precursor condition to esophageal adenocarcinomas (EAC). EAC are usually diagnosed at an advanced stage and are difficult to treat. Finding Barrett's esophagus, a sign of risk, could help patients by giving physicians a chance to intervene early. The technology was made more widely available with a special Food and Drug Administration (FDA) designation in February 2020.
These innovations were developed with support from programs of the National Cancer Institute (NCI) and with collaboration among oncologists, gastroenterologists, and pathologists. The EsoCheck™ device was recently named a 2020 Edison Award™ Silver Winner as one of the year's most significant innovations in the Medical Testing category.
The 15% to 30% of people in the United States who have chronic gastro-esophageal reflux disease, more commonly known as GERD, are at most risk for Barrett's esophagus. Those with chronic GERD are recommended to have an endoscopy to check for pathologic features of the condition. Endoscopy is an invasive procedure requiring sedation and a loss of a day at work for the patient and the necessary companion. After diagnosis, patients are followed longitudinally with more endoscopic exams and at the first signs of the condition worsening, the affected tissues can be removed to prevent the development of cancer.
"Only about 7% of patients who are diagnosed with esophageal adenocarcinoma have a prior diagnosis of Barrett's esophagus, indicating that most have never had that recommended endoscopy," said Ellen Richmond, M.S., G.N.P.-B.C., a program director in the NCI Division of Cancer Prevention (DCP) for the Barrett's Esophagus Translational Research Network (BETRNet). "Symptoms of EAC often develop only when the cancer has reached late stages. We needed a way to safely screen a much broader segment of the population that is quick, non-invasive, inexpensive, and easily administered to those who are currently unscreened."
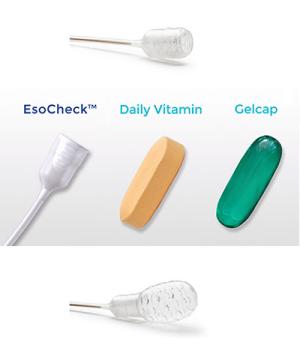
The EsoCheck device (from Top to Bottom), when swallowed, in comparison to a vitamin or capsule, and when inflated.
The conception and development of a biomarker based non-endoscopic technology for detecting Barrett's esophagus happened through support from DCP's Early Detection Research Network (EDRN) and BETRNet programs, and the Division of Cancer Treatment and Diagnosis' Translational Research Program (SPOREs).
To address the challenge of finding a better way to diagnose Barrett's esophagus, Sanford Markowitz, M.D., Ph.D., and his co-investigators, Amitabh Chak, M.D., and Joseph Willis, M.D., at Case Western Reserve University in Cleveland, embarked on research on two fronts: to identify robust DNA markers of Barrett's esophagus and then to find a way to get samples for testing without endoscopy.
The team had previously discovered that the VIM gene, which codes for vimentin (a structural protein that is overexpressed in multiple cancers), is altered by methylation in the development of colon cancer. These changes in the DNA can be detected in the stool of humans with colon cancer. This discovery was proof that gastrointestinal cancers can be detected through this specific DNA change by a biomarker test, instead of through an invasive examination.
Knowing that the tissues along the gastrointestinal tract share many similarities, they tested for vimentin methylation in archived tissue samples of EAC and Barrett's esophagus. They found that the marker was present in 90% of EAC and also in 90% of Barrett's esophagus samples—an ideal biomarker of cancer development in the esophagus. Later, they demonstrated that the biomarker could be detected in samples from brushings of the esophagus taken during endoscopy in those with EAC and those with Barrett's.
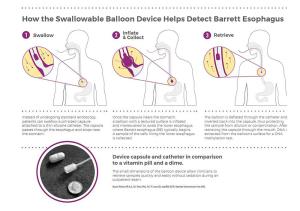
In order to collect samples without requiring endoscopy, the Case Western Reserve investigators developed a "swallowable" balloon-based device to enable less invasive targeted sampling of the lower esophagus (see sidebar). The collected cells are then tested with the methylated DNA biomarker.
How EsoCheck™ Works
"One of the keys to this early detection device is the ability to sample tissue from only the lower esophagus in a noninvasive manner," said Sudhir Srivastava, Ph.D., M.P.H., chief of the NCI DCP Cancer Biomarkers Research Group, which supported this research through the EDRN. "EsoCheck fills that need."
In the first-in-human clinical trial, the combination of the balloon-based device and a DNA test of methylated vimentin plus methylated CCNA1, showed over 90% sensitivity (correctly identifying people with Barrett's esophagus) and 90% specificity (correctly identifying people without Barrett's esophagus.) This showed that the less invasive device and new DNA test were as accurate in finding the condition as testing by endoscopy.
The DNA test was further refined and moved into use at the University Hospitals Cleveland Medical Center Pathology Department. The technology and test were licensed to Lucid Diagnostics for commercial development with the swallowable balloon device commercialized as EsoCheck™ and the methylated DNA diagnostic test as EsoGuard™.
The EsoCheck™ device was awarded FDA 510K clearance in 2019, demonstrating it as safe and effective prior to marketing. In February 2020, FDA gave the test and device combination an FDA Breakthrough Device Designation, allowing patients faster access to the technology because it is more effective in finding a "life-threatening or irreversibly debilitating human disease or condition."
"The EsoCheck/EsoGuard combination shows how the NCI-sponsored biomarker discovery and validation process can lead to new discoveries, new technologies, and new clinical products," said Markowitz. "Ultimately, critical collaborations are vital to creating products that lead to preventing deaths through earlier detection of precancers and cancers that in later stages are largely untreatable and cause death."
Several large multicenter clinical trials are evaluating the combination's efficacy, safety, and patient tolerability in patients with Barrett's esophagus, who have had previous endoscopies, or in people with GERD who have not. The technology is also being further validated in a LucidDx sponsored national trial aimed at winning FDA clearance for the EsoGuard DNA diagnostic assay.
Clinical Trials Under Way Using EsoCheck and EsoGuard
- Genetic Determinants of Barrett's Esophagus and Esophageal Adenocarcinoma (FBE) by BETRNet
- Efficacy of EsoGuard on Samples Collected Using EsoCheck Versus EGD for the Diagnosis of BE (ESOGUARDBE1) by Lucid Diagnostics Inc.
- Efficacy of EsoGuard Assay on Esophageal Surface Cells Collected With EsoCheck vs EGD for the Diagnosis of BE or EAC (ESOGUARDBE2) by Lucid Diagnostics Inc.
Key Publications
Chen WD, Han ZJ, Skoletsky J, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst 97:1124-1132, 2005.
Moinova HR, Leidner RS, Ravi L, et al. Aberrant vimentin methylation is characteristic of upper gastrointestinal pathologies. Cancer Epidemiol Biomarkers Prev. 2012 April;21(4): 594-600. Epub 2012/02/09 PNCID: PMC3454489.
Moinova HR, LaFromboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett's esophagus. Sci Transl Med 2018 Jan 17;10(424).
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "New Technology Gives Patients Access to a 5-Minute, Office-Based Test to Identify Risk for Esophageal Cancer was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.
