Past Presentations
Pre-Application Webinars
The NCI Division of Cancer Prevention held pre-application interactive webinars for funding opportunities for the Cancer Screening Research Network (CSRN). The first webinar provides an overview of the Network, and addresses all three grants. There are three subsequent RFA-specific webinars. NCI staff discuss the funding opportunities and answer questions from prospective applicants. Participants were asked to submit their questions ahead of time to CSRN@nih.gov by the Wednesday preceding the webinar of interest by 11:59 PM EST. Participation in the webinars was optional.
RFA-CA-23-020: NCI Cancer Screening Research Network: ACCrual, Enrollment, and Screening Sites (ACCESS) Hub
Date: Friday, December 16, 2022
Runtime: 00:58:33
RFA-CA-23-021: NCI Cancer Screening Research Network: Statistics and Data Management Center
Date: Friday, December 16, 2022
Runtime: 00:34:34
RFA-CA-23-022: NCI Cancer Screening Research Network: Coordinating and Communication Center (UG1 Clinical Trial Required)
Date: Friday, December 16, 2022
Runtime: 00:27:12
All CSRN Components RFA-CA-23-020, RFA-CA-23-021, RFA-CA-23-022
Date: Friday, December 9, 2022
Runtime: 00:58:27
Notice of Intent to Publish the CSRN Funding Opportunity Presentation

In response to questions coming from the Notice of Intent to Publish, a shorter description of the BSA presentation focused on the CSRN rationale and structure is posted for those investigators interested in considering applications to CSRN.
Date: Monday, November 14, 2022
NCI Board of Scientific Advisors Presentation
On Wednesday, June 15, 2022, the Division of Cancer Prevention presented a request to fund an RFA entitled "Cancer Screening Network to Evaluate Multi-Cancer Detection Assays for Clinical Utility in Cancer Screening".
Date: Wednesday, June 15, 2022
Time: 2:45 p.m. ET
Meeting agenda (PDF, 211 KB)
A recording of this presentation available at https://videocast.nih.gov/watch=45621
Application Information/Frequently Asked Questions (FAQs)
Questions and answers about the Cancer Screening Research Network (CSRN) are grouped into 6 categories and are available on individual pages:
- General Application FAQs
- ACCrual, Enrollment, and Screening Sites (ACCESS) Hub FAQs
- Coordinating and Communication Center (CCC) FAQs
- Statistics and Data Management Center (SDMC) FAQs
- Types of Studies FAQs
- Vanguard Study FAQs
For additional questions, please contact CSRN@nih.gov.
Relevant Links
Multi-Cancer Detection Notices
- NOT-CA-23-055: NCI Virtual Workshop to Engage Multi-Cancer Detection (MCD) Assay Developers
- NOT-CA-22-033: Seeking Input from Multi-Cancer Early Detection Test Developers on Readiness for Participation in an NCI-Sponsored Clinical Utility Randomized Controlled Screening Trial
CSRN Requests for Applications (RFAs)
- RFA-CA-23-020: Cancer Screening Research Network: ACCrual, Enrollment, and Screening Sites (ACCESS) Hub
- RFA-CA-23-021: Cancer Screening Research Network: Statistics and Data Management Center
- RFA-CA-23-022: Cancer Screening Research Network: Coordinating and Communication Center
Use of NCI Clinical Trials Infrastructure
The Cancer Screening Research Network (CSRN) will use the NCI Clinical Trials Infrastructure which includes a variety of integrated electronic systems, applications, and processes, which together facilitate the conduct of cancer clinical trials.
This infrastructure, called the Clinical Oncology Research Enterprise (CORE) is managed by NCI’s Cancer Therapy and Evaluation Program (CTEP). This infrastructure provides the functions necessary to support the CSRN, including:
- Investigator and institution registration
- Study administration and logistics
- Electronic clinical data capture and reporting
- Correlative study data
- Regulatory monitoring and reporting, including adverse event reporting and auditing
- Data quality and control
- Information security
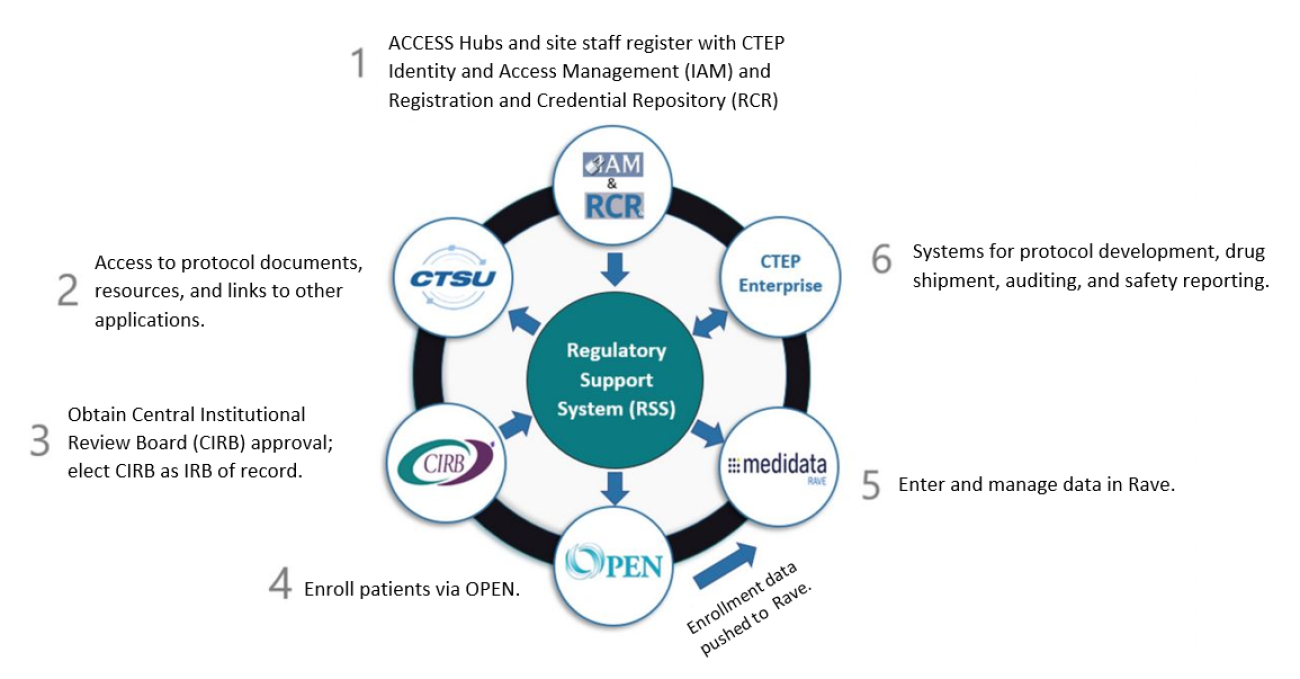
CTEP-Identity and Access Management (CTEP-IAM)
- Investigators and research staff register for an account that enables access to the other applications.
Cancer Trials Support Unit (CTSU)
- Provides the CSRN with a suite of integrated systems to coordinate the administrative, regulatory, and logistical functions, and provide information technology support. It offers a variety of services, including institution and person roster support; website support for posting of protocols and other information; and links to other services.
- Oncology Patient Enrollment Network (OPEN)
- Linked applications for participant enrollment. Data will be automatically transferred to Rave.
- Linked applications for participant enrollment. Data will be automatically transferred to Rave.
- Regulatory Support Services (RSS)
- Serves as a centralized repository for the regulatory documents for all NCI-supported multi-center clinical trials. The RSS provides a streamlined and comprehensive approach to collecting and maintaining site registration, person, and institution documentation essential to the management of clinical trials.
- Oncology Patient Enrollment Network (OPEN)
- An application for data entry, data analysis, and clinical trial management.
- Targeted Source Data Verification (TSDV)
- Enables the electronic capture of Source Data Verification activity in Rave. The TSDV helps in capturing the audit trail of the audit activities for a participant and provides additional transparency to the site audit process.
NCI Central Institutional Review Board (CIRB)
- Conducts IRB review for NCI-sponsored trials, including CSRN trials and studies. It meets the federal regulatory requirements and NIH policy for single IRB review.
- An application for integrated clinical trials management and reporting, including Serious Adverse Event (SAE) reporting through the CTEP-Adverse Event Reporting System (CTEP-AERS), ordering of investigational agents, and trial monitoring/audits.
Registration and Credentialing Repository (RCR)
- An application used for the collection and management of NCI annual registration documents to ultimately generate an on-demand site- and protocol-specific registration report, based on study activities assigned on the trial’s Delegation of Tasks Log. All investigators who participate in NCI-supported clinical trials networks need to register with the RCR and renew their registration annually.
The NCI Clinical Trials Reporting Program (CTRP)
- Provides a comprehensive database of information on all NCI supported clinical trials open to accrual. It helps identify gaps and duplicate studies in clinical research, facilitates clinical trial prioritization, and standardizes trial data capture and sharing.
NCI Cancer Data Access System (CDAS)
- Will serve as the archive for completed CSRN trial data.
- Allows investigators to request access to data, images, or biospecimens from NCI studies. This is an effort to make data more publicly accessible to the research community.
Research Performance Progress Report (RPPR)
- An application used by NIH grant recipients to submit progress reports to NIH on their grant awards.
DCP Procedures
Procedure for Concept and Protocol Submission to NCI
Concepts developed by the CSRN will be submitted to the DCP Protocol Information Office (PIO). These will be assessed for completeness and forwarded to the NCI Cancer Prevention Steering Committee for concept review. If a concept is approved, the CSRN will develop a protocol and submit it to the PIO. The PIO will assess the protocol for completeness and schedule it for review by the DCP Protocol Safety Review Committee. After DCP approval, the protocol will be forwarded to the CIRB for review. A protocol must receive approval from DCP, the CIRB, and the CTSU to receive the final DCP letter of approval.
DCP Screening Protocol Requirements
The CSRN will adhere to the DCP Cancer Screening Clinical Trial Requirements, as approved by DCP on July 29, 2022. The requirements specify items to be incorporated into the protocol document and processes that DCP will employ to monitor accrual progress.
DCP Communication Requirements
Participant-facing information requires CIRB review. Communications to other audiences such as media releases, meeting abstracts, scientific papers, professional educational materials, social media, etc. will require DCP review (submitted via csrn@nih.gov).
Preliminary Design for Large-Scale Trial
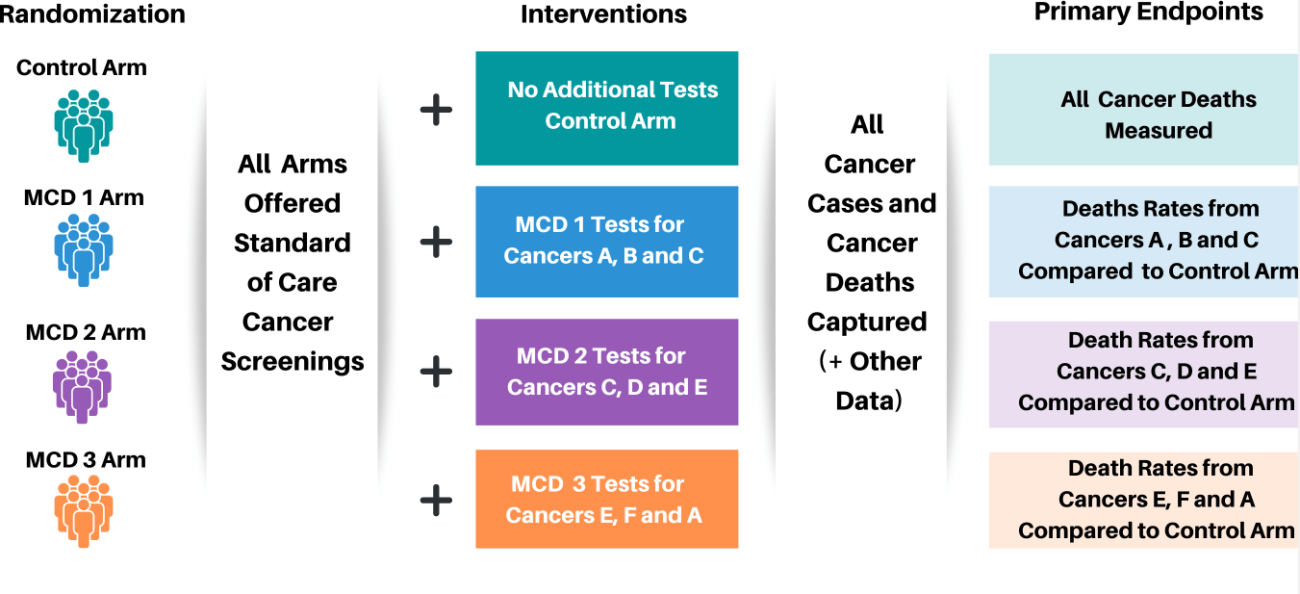
The preliminary design for a large-scale trial will include a control arm that receives no additional tests, an arm that receives one multi-cancer detection test (MCD) that will test for cancers a, b, and c (called MCD 1 arm); a second arm that receives a second MCD test that test for cancers c, d, and e (called MCD 2 arm); and a third arm that receives a third MCD test that test for cancers e, f, and a (called MCD 3 arm).
All cancer cases and cancer deaths will be captured plus other data for all arms of this study.
The primary endpoints for the control arm are all cancer deaths measured.
The primary endpoints for the MCD 1 arm are death rates from cancers a, b, and c compared to the control arm.
The primary endpoints for MCD 2 arm are death rates from cancers c, d, and e compared to the control arm.
The primary endpoints for the MCD 3 arm are death rates from cancers e, f, and a compared to the control arm.
