The CSRN launched a pilot study, called the Vanguard Study, to address the feasibility of using multi-cancer detection (MCD) tests in future randomized controlled trials (RCTs). The Vanguard Study will enroll up to 24,000 people to inform the design of a much larger RCT that will evaluate whether the benefits of using MCD tests to screen for cancer outweigh the harms, and whether they can detect cancer early in a way that reduces deaths.
Study Status: Actively Recruiting Participants
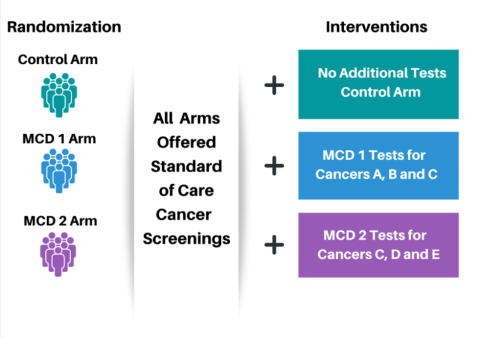
Randomization will be into a Control Arm, and two separate MCD Arms, labelled MCD 1 and MCD 2 in the graphic. Participants in all arms will be encouraged to receive standard of care cancer screenings.
The National Cancer Institute (NCI) has selected two assays for the Vanguard Study. The assays were selected based on a formal process that included a public workshop to engage multi-cancer detection assay developers. The assays selected, in alphabetical order, are:
These two assays will not be compared to each other but will be compared to cancers detected in the Control Arm. The companies are providing the assays without cost to the study participants.
The Control Arm participants will have blood drawn but have no additional testing at this time.
The objectives of the Vanguard Study are to:
- assess participant willingness for randomization;
- determine adherence to testing and diagnostic follow-up;
- evaluate feasibility of protocol-defined diagnostic workflows;
- determine reliability and timeliness of blood specimen testing and return by MCD companies; and
- identify facilitators and barriers to recruitment/retention/compliance of diverse participant groups.
Learn more about The Vanguard Study on Multi-Cancer Detection Tests.
