Date Posted, by Vikrant Sahasrabuddhe, M.B.B.S., M.P.H., Dr.P.H.

Members of the US-Latin American-Caribbean Clinical Trials Network and National Cancer Institute researchers work together to improve and optimize approaches for prevention of HPV-related cancers in people living with HIV.
Cervical cancer is highly preventable, yet it is not fully prevented. Significant advancements in our understanding of the human papillomavirus (HPV)-linked development of cervical cancer have created highly effective clinical tools for primary prevention (HPV vaccines) and secondary prevention (screening and treatment of precancerous lesions). Despite this, more than half a million women are diagnosed and over 300,000 women die of cervical cancer every year globally. Universal approaches for the delivery and use of clinical prevention interventions for cervical cancer are not standardized. In places where healthcare resources are limited, the gap between knowledge and action is even greater. The disconnect between discovery and delivery is most stark in the context of women living with HIV, who constitute over half of the more than 37 million people living with HIV globally.
Women with HIV have a three to five times higher risk of cervical cancer due to immunosuppression. Facing a dual burden of HIV disease and HPV/cervical cancer, these women are younger than the average age at cancer diagnosis. Their disease has a more aggressive clinical course and is less responsive to treatment. Access to affordable HIV treatment has expanded globally over the past several years, and millions of women living with HIV are living longer lives. Yet with the absence of efficient clinical prevention services, these women continue to be at risk of dying from cervical cancer. In addition to cervical cancer, people living with HIV are also at risk for other HPV-related cancers, including anal cancer, other lower genital tract cancers, and oropharyngeal cancers. Despite the substantial need, clinical practice guidelines and public health recommendations for the prevention of cervical cancer and other HPV-related cancers in people living with HIV have not been refined. In areas with low resources there is already a lack of, or sub-optimal use of, traditional prevention-oriented healthcare services.
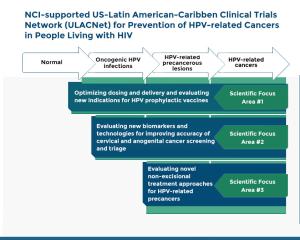
The National Cancer Institute (NCI) Division of Cancer Prevention (DCP) has originated several initiatives to expand the evidence base on clinical prevention interventions for cervical cancer and HPV-related cancers in people living with HIV. These activities cover the continuum of prevention interventions for HPV-related cancers, including:
- optimizing dose and delivery, and evaluating new indications for HPV prophylactic vaccines for adults and children living with HIV;
- evaluating new biomarkers and imaging technologies for improving accuracy of cervical and anogenital cancer screening and triage for people living with HIV; and
- evaluating novel non-excisional treatments, including immunotherapeutic approaches and topically applied agents for treatment of HPV-related anogenital precancerous lesions in people living with HIV.
In Fall 2019, the United States-Latin American-Caribbean Clinical Trials Network (ULACNet) was launched. ULACNet is an international collaborative research network that brings together institutions in the US and their counterparts in Latin American and Caribbean countries. Funded via grants, investigators in three ULACNet Partnership Centers collaborate with the NCI to design and conduct clinical trials of prevention interventions for HPV-related cancers for women, men, and children living with HIV. Headed by DCP, ULACNet includes partnerships with NCI colleagues from the Office of HIV/AIDS Malignancy, Center for Global Health, and the Division of Cancer Epidemiology and Genetics, as well as with the National Institute of Dental and Craniofacial Research (NIDCR), and has clinical trial sites in the US, Mexico, Puerto Rico, Brazil, Peru, and Dominican Republic.
DCP also supports a portfolio of investigator-initiated clinical trials and prevention research studies in this scientific area. In addition, DCP collaborates with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to support a US-based multicenter clinical study in the Pediatric HIV/AIDS Cohort Study (PHACS) Network focused on evaluating HPV disease outcomes in perinatally-HIV-infected children in the US as they transition to young adulthood.
To accelerate the translation of evidence-based interventions to clinical and public health practice for women living with HIV, DCP's activities also include co-leading collaborative studies in Zambia and India, as well as providing technical advice to the US State Department's President's Emergency Plan for AIDS Relief (PEPFAR) and the World Health Organization (WHO).
Building on NCI's long history of developing life-saving interventions (PDF, 112 KB) for cervical cancer and HPV-related cancers, current efforts are expected to produce critical data for innovative interventions to prevent HPV-related cancers among people living with HIV. In addition, biologic insights into viral-host interactions and immunologic contributions to cancer development will inform improvements in clinical care options for people living with HIV globally.
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "Optimizing Clinical Interventions for Prevention of Cervical Cancer and HPV-related Cancers in People Living with HIV was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.