Date Posted
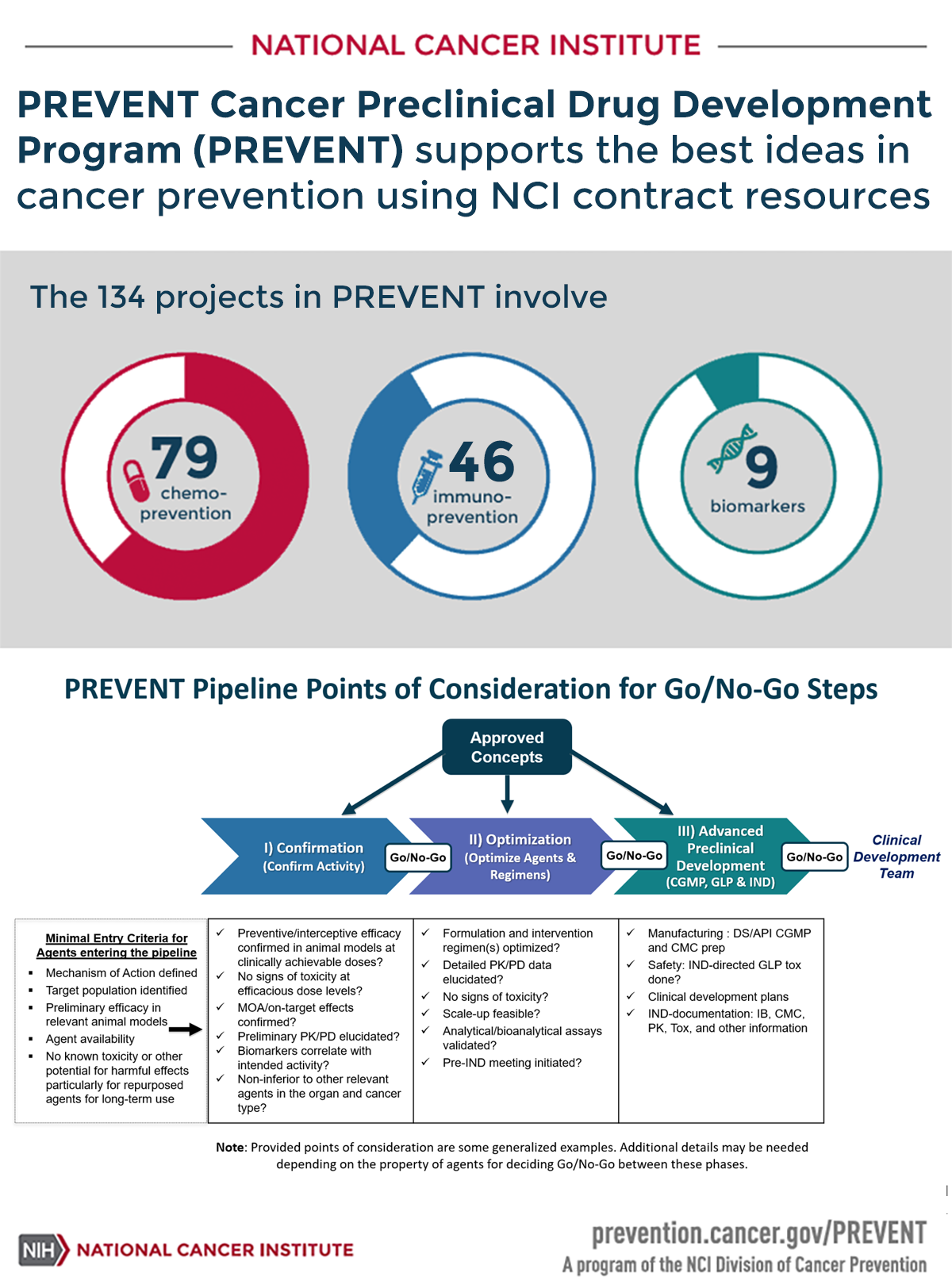
PREVENT Cancer Preclinical Drug Development Program (PREVENT) supports the best ideas in cancer prevention using NCI contract resources
The 134 projects in PREVENT involve:
- 79 Chemoprevention
- 46 Immunoprevention
- 9 Biomarkers
Preclinical Drug Development Pipelines involves:
- Proof of Concept
- 34 Chemoprevention
- 33 Immunoprevention
- 9 Biomarkers
- Secondary Testing
- 34 Chemoprevention
- 4 Immunoprevention
- Advances Preclinical Development
- 11 Chemoprevention
- 9 Immunoprevention
PREVENT Pipeline Points of Considerations for Go/No-Go Steps
Approved Concepts
Minimal Entry Criteria for Agents entering the pipeline
- Mechanism of Action defined
- Target population identified
- Preliminary efficacy in relevant animal models
- Agent availability
- No known toxicity or other potential for harmful effects particularly for repurposed agents for long-term use
PREVENT has flexible entry points. Approved concepts can enter the pipeline at any one of the following stages. Points of consideration for Go/No-go steps are provided under each stage.
NOTE: Provided points of consideration are some generalized examples. Additional details may be needed depending on the property of agents for deciding Go/No-Go between these phases.
Confirmation (Confirm Activity)
- Preventive/interceptive efficacy confirmed in animal models at clinically achievable doses?
- No signs of toxicity at efficacious dose levels?
- MOA/on-target effects confirmed?
- Preliminary PK/PD elucidated?
- Biomarkers correlate with intended activity?
- Non-inferior to other relevant agents in the organ and cancer type?
Optimization (Optimize Agents & Regimens)
- Formulation and intervention regimen(s) optimized?
- Detailed PK/PD data elucidated?
- No signs of toxicity?
- Scale-up feasible?
- Analytical/bioanalytical assays validated?
- Pre-IND meeting initiated?
Advanced Preclinical Development (CGMP, GLP & IND)
- Manufacturing : DS/API CGMP and CMC prep
- Safety: IND-directed GLP tox done?
- Clinical development plans
- IND-documentation: IB, CMC, PK, Tox, and other information
