Cancer treatment-related cardiotoxicity is damage to the heart and/or cardiovascular system (including heart valves and vessels) that can occur during or after cancer treatment (see more in this blog post). Chemotherapy and radiation therapy, as well as newer forms of cancer treatment like immune system therapies, can cause or worsen cardiovascular side effects, including high blood pressure, abnormal heart rhythms, and heart failure. Cardiotoxicity symptoms during cancer treatment can lead to interruption or stopping of treatment, reducing its effectiveness. Symptoms may also arise years after cancer treatment.
As improvements in treatment have led to more cancer patients surviving longer, research is focusing on how side effects like cardiotoxicity affect cancer survivors. Researchers are investigating the health issues around cardiotoxicity, who is at risk, and how to reduce that risk.
On This Page
- All Heading 2s will automatically be pulled in to this list.
- Do not edit the content on this template.
Case Report Forms
Download the Common Data Elements for Cardio-Oncology Case Report Forms
Increasing Focus on Cardio-Oncology
In response to this growing need, starting in 2013, the National Institutes of Health has increased its support of cancer treatment-related cardiotoxicity research through the funding of grants and coordination of internal and external working groups.
Key milestones in this effort include:
- March 2013: The National Cancer Institute (NCI) and the National Heart, Lung, and Blood Institute (NHLBI) jointly sponsored a workshop to gather experts in this area. Together, lead cardiology and oncology researchers from academia, government, and industry, along with patient advocates, worked to identify the scope of the problem and gaps in research. (The meeting summary was published in the Journal of the National Cancer Institute.) Over 40 scientific research gaps were identified.
- January 2014: As a result of the 2013 workshop, an NCI Community Oncology Cardiotoxicity Task Force was formed. NCI’s Division of Cancer Prevention (DCP) initiated the multidisciplinary Task Force to coordinate study designs and research activities across the NCI Community Oncology Research Program (NCORP). The Task Force met regularly to discuss latest research findings, identify priorities for new investigations, and challenge each other to create the best study designs.
- June 2015: A workshop of investigators conducting cardiotoxicity studies sponsored by DCP highlighted the need for quality control of echocardiogram findings in cardiotoxicity studies. Echocardiogram, a non-invasive technology that is widely available, is commonly used to assess cardiac function while cancer patients receive therapy. The results from this workshop suggested that central review is preferred to promote consistent results reporting for echocardiogram-defined primary endpoints in clinical trials and studies.
- December 2016: The Cancer Moonshot was launched through the 21st Century Cures Act. Research into cardiotoxicity from cancer treatment directly pertains to the Cancer Moonshot priority area of minimizing cancer treatment’s debilitating side effects. (See more in this article on The Impact of the Cancer Moonshot on Cardio-Oncology Science).
- June 2018: NCI and NHLBI sponsored a follow-up conference on treatment-related cardiotoxicity. The goal was to bring together experts in multiple disciplines within cardiology and oncology to highlight and discuss progress, explore ongoing work, and redefine and update research priorities since the 2013 workshop. (See the workshop summary in Current Oncology Reports.)
The identification of gaps in research and increased collaboration between cardiology and oncology investigators as a result of these workshops led to the issuance of funding opportunity announcements (FOAs) and an increased number of funded projects in the basic and translational science of cardio-oncology. (Learn more about the evolving design of cardio-oncology studies.)
Feature Article
Spectrum of National Institutes of Health-Funded Research in Cardio-Oncology: A Basic, Clinical, and Observational Science Perspective
In 2022, in the journal Heart Failure Clinics, the cardio-oncology team from NCI and NHLBI wrote about the decade-plus NIH-supported cardio-oncology research being conducted, and its importance.
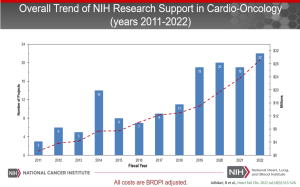
NCI-Supported Funding Opportunities
In 2021, NCI and NHLBI issued a Notice of Special Interest (NOSI) on “Improving Outcomes in Cancer Treatment-Related Cardiotoxicity.” Its purpose is to foster collaboration among oncology and cardiology researchers and encourage innovative approaches to:
- Improve understanding of the mechanisms of cardiotoxicity attributed to cancer treatment to develop new therapeutic strategies for cardio-protection
- Develop pre-clinical models to identify potential adverse cardiovascular outcomes in drug development studies
- Differentiate and quantify characteristics associated with short- and long-term cardiotoxicity
- Develop and test methods to detect early-onset cardiovascular dysfunction
- Develop, test, and evaluate novel approaches to prevention, management, and care coordination strategies for cardiotoxicity
A NOSI is an announcement of an interest in receiving grant applications on a specific topic of research. Each NOSI includes a list of funding opportunity announcements to which researchers may apply. This NOSI expires November 6, 2024.
DCP’s Research Portfolio includes these important studies
- Children's Oncology Group (ALTE 11C2), Effects of Dexrazoxane Hydrochloride on Biomarkers Associated with Cardiomyopathy and Heart Failure after Cancer Treatment (HEART) (NCT01790152)
- Children's Oncology Group (ALTE 1621), Carvedilol in Preventing Heart Failure in Childhood Cancer Survivors (NCT02717507)
- St. Jude Children’s Research Hospital, An Open-Label Intervention Trial to Reduce Senescence and Improve Frailty in Adult Survivors of Childhood Cancer (NCT04733534, U01 STAR Act funded)
- SWOG (S1501) Prospective Evaluation of Carvedilol in Prevention of Cardiac Toxicity in Patients with Metastatic HER-2+ Breast Cancer, Phase III (NCT03418961)
- Wake Forest (WFU 97415) Understanding and Predicting Breast Cancer Events after Treatment (UPBEAT) (NCT02791581)
In addition, numerous investigator-initiated grants in cardiotoxicity are overseen by DCP Program Directors.
Closed to Accrual
- MD Anderson Cancer Center (MDA-2007-0914), A Multicenter Study in Patients Undergoing Anthracycline-Based Chemotherapy to Assess the Effectiveness of Using Biomarkers to Detect and Identify Cardiotoxicity and Describe Treatment (PREDICT) (see related publication)
- SunCoast Community Clinical Oncology Programs Research Base at University of South Florida (SCUSF 0806), Lisinopril or Coreg vs. Placebo in Reducing Side Effects in Women with Breast Cancer Receiving Trastuzumab (see related publication)
- Wake Forest University Health Sciences (WFU 98213), Preventing Anthracycline Cardiovascular Toxicity with Statins (PREVENT) (NCT01988571)
Related Outside Links
- American College of Cardiology (ACC) - Cardio-Oncology Section
- American Society of Clinical Oncology (ASCO) - Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline
- Canadian Cardiac Oncology Network (CCON)
- Cardio-Oncology journal
- CardioSmart patient education website - joint effort of ACC and the Eastern Cooperative Group-American College of Imaging Network (ECOG-ACRIN)
- International Cardio-Oncology Society (ICOS)
- JACC: CardioOncology
