
-
Last Mile Initiative
-
The SHIP Trial
Latest News and Information
-
 FDA Approves HPV Tests That Allow for Self-Collection in a Health Care Setting
FDA Approves HPV Tests That Allow for Self-Collection in a Health Care SettingOn May 14, the Food and Drug Administration (FDA) expanded the approvals of two tests that detect cancer-causing types of human papillomavirus (HPV) in the cervix. Both tests are used as part of screening for cervical cancer.
-
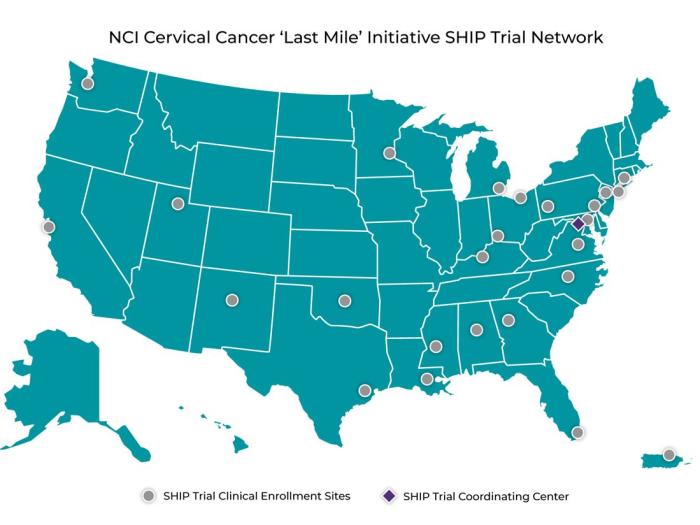 NCI Launches Network to Study Self-Collection for HPV Testing to Prevent Cervical Cancer
NCI Launches Network to Study Self-Collection for HPV Testing to Prevent Cervical Cancer‘Last Mile’ Initiative Will Test Alternative Approach to Screening Through the SHIP Trial Network
-
 Cancer Healthcast: NCI ‘Last Mile’ Initiative Innovates Self-Sampling for HPV Screening
Cancer Healthcast: NCI ‘Last Mile’ Initiative Innovates Self-Sampling for HPV ScreeningNew self-sampling technologies screening for HPV could help increase access and reduce cervical cancer among underserved and high-burden populations.
Contact Information
Email: CervicalCancerLastMile@nih.gov
Phone: 240-276-7332
Fax: 240-276-7828
