Skip to main content
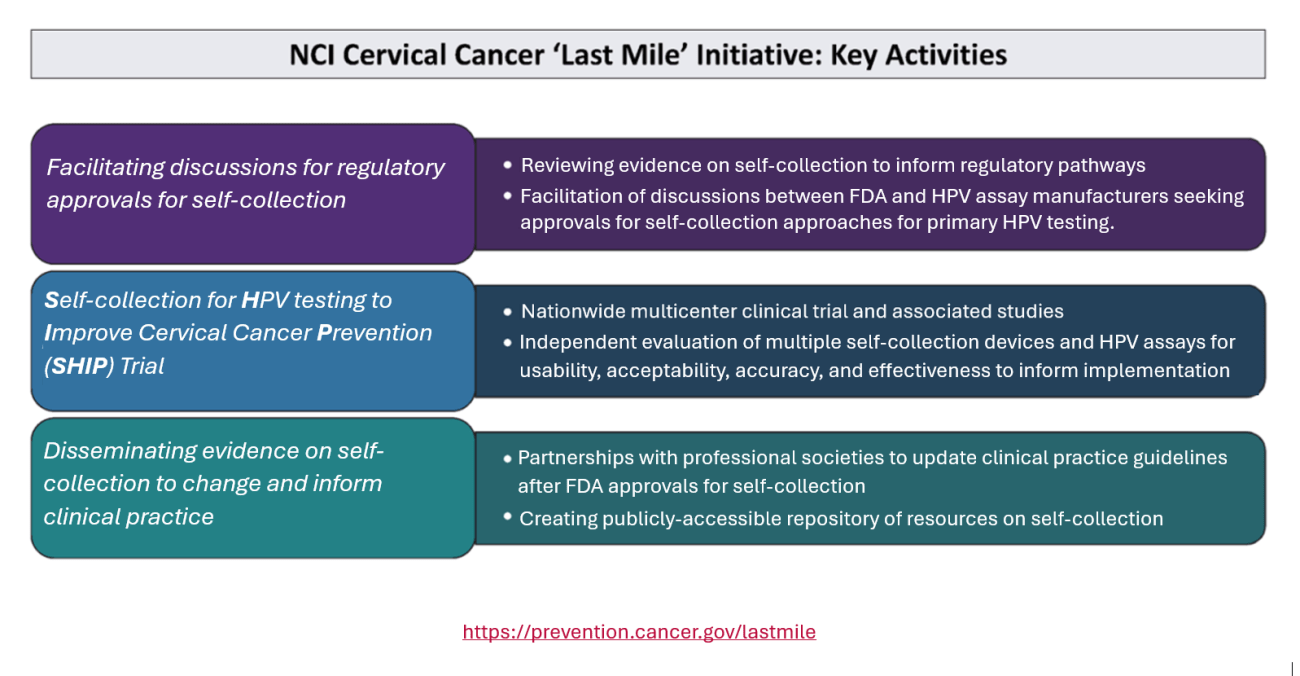
NCI Cervical Cancer ‘Last Mile’ Initiative (LMI)
Facilitating discussions for regulatory approvals for self-collection
- Reviewing evidence on self-collection to inform regulatory pathways
- Facilitation of discussions between FDA and HPV assay manufacturers seeking approvals for self-collection approaches for primary HPV testing
Self-collection for HPV testing to Improve Cervical Cancer Prevention (SHIP) Trial
- Nationwide multicenter clinical trial and associated studies
- Independent evaluation of multiple self-collection devices and HPV assays for usability, acceptability, accuracy, and effectiveness to inform implementation
Disseminating evidence on self-collection to change and inform clinical practice
- Partnerships with professional societies to update clinical practice guidelines after FDA approvals for self-collection
- Creating publicly-accessible repository of resources on self-collection