Date Posted, by Philip E. Castle, Ph.D., M.P.H.

DCP Director Philip E. Castle, Ph.D., M.P.H.
In the month of October, breast cancer awareness is the health message that looms large. Yet, researchers across the National Cancer Institute and across the world are focused on breast cancer all the time. In the United States, more than 275,000 women are expected to be diagnosed with breast cancer this year and more than 3.5 million are breast cancer survivors.
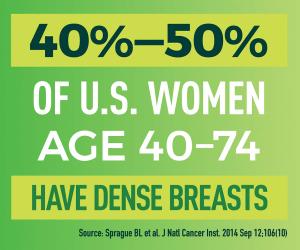
The best breast cancer, however, is the one that never happens. That is where the prevention and detection research supported by the NCI Division of Cancer Prevention (DCP) is making a difference. We know that many things can influence a woman’s risk of developing breast cancer, some of which can be modified, like hormone medication use or alcohol intake, and some of which cannot, like inherited genes or breast density. We also know that breast cancer takes years to develop, giving us years to intercept to change a woman’s chance of developing this disease.
Here are five of the many breast cancer research projects the division is supporting:
On This Page
- All Headings will automatically be pulled in to this list.
- Do not edit the content on this template.
Topical Tamoxifen
Oral tamoxifen was shown to decrease the incidence of breast cancer in women at increased risk in 1998. Using the drug as a pill has had side effects like increased risk of blood clots and endometrial cancer. DCP will be completing accrual this month for a breast density study to examine the impact of 4-hydroxytamoxifen gel compared to placebo to reduce breast density. This is one of four trials that DCP is funding to move 4-hydoxytamoxifen forward from Phase II to Phase III studies.
Researchers are searching for evidence of breast cancer in the blood of patients. This is also known as a liquid biopsy.
Breast Cancer Liquid Biopsy Project
At the University of Miami School of Medicine, Ashutosh Agarawal, Ph.D., and his team are developing a diagnostic tool that captures, enumerates, and analyzes circulating tumor cells and associated cancer cells from blood. The platform is being evaluated to predict recurrence/survival in early stage breast cancer patients. The tool could become a platform for screening of novel drugs and in precision cancer management.
Development and Clinical Implementation of an Artificial Intelligence Tool to Predict Risk of Upgrade of Ductal Carcinoma In Situ (DCIS)
At Massachusetts General Hospital, Manisha Bahl, M.D., is working in the laboratory of Regina Barzilay, Ph.D., at the Massachusetts Institute of Technology, to develop a robust AI tool that incorporates clinical data, mammographic imaging, and biopsy histopathology slides for pre-operatively predicting the risk of concurrent invasive cancer in women with DCIS. Every year, more than 60,000 women are diagnosed with DCIS and undergo an aggressive treatment regimen involving surgery, radiation, and hormonal therapy. Development of a highly reliable prognostic tool could identify the subset of women with DCIS who may not need such aggressive treatment.
Comparison of Molecular Breast Imaging and Digital Breast Tomosynthesis for Screening in Women with Dense Breasts (MBI-DBT)
Deborah Rhodes, M.D., and her team at the Mayo Clinic are running a study of molecular breast imaging (MBI), a relatively low-cost functional breast imaging technique, compared to Digital Breast Tomosynthesis (DBT), the standard anatomic breast cancer screening technique in women with dense breasts. Data from the trial will help determine how women with dense breasts can get optimal screening tests for breast cancer.
Tomosynthesis Mammographic Imaging Screening Trial (TMIST)
TMIST is a large NCI-funded trial comparing two techniques used for mammograms: tomosynthesis, often called 3D mammography, and standard 2D digital mammography. The 164,000-person trial is designed to resolve an important unknown: whether tomosynthesis has a meaningful impact on the detection of potentially life-threatening breast cancers. The study’s principal investigator is Etta Pisano, M.D., of Beth Israel Deaconess Medical Center in Boston and chief science officer of the American College of Radiology. The study is enrolling at more than 100 sites across the U.S., Canada, and in South America. Because NCI places a high priority on answering the questions about optimal breast cancer screening and management, a working group has been named to review accrual to TMIST. During this review, the trial is fully open, both for women who are already participating and those who are interested in enrolling. NCI thanks the women who are and will be enrolled in TMIST for their participation.
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "Projects Making Progress in Breast Cancer Detection and Prevention was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.