Date Posted, by Howard L. Parnes, M.D.

In the path toward understanding the development and ultimate prevention of prostate cancer, researchers are studying how to keep low-grade, early stage prostate cancer being monitored on Active Surveillance from progressing to disease that needs definitive treatment.
Watchful Waiting vs Active Surveillance – What does it Mean for Men with Prostate Cancer?
"Watchful Waiting" is an approach reserved for prostate cancer patients with limited life-expectancies who are unlikely to benefit from definitive therapy, e.g., radical surgery or radiation. It consists of PSA and symptom monitoring (once or twice a year) until metastatic disease occurs at which time palliative hormone therapy is administered to relieve symptoms and improve quality of life.
"Active Surveillance" is an approach reserved for men with low or favorable intermediate-risk prostate cancer who wish to avoid or delay definitive therapy. It consists of monitoring with PSA and digital rectal exam (every 6 months) and follow-up prostate biopsies (every 1 to 3 years). In contrast to Watchful Waiting, definitive therapy with curative intent is offered at the time of disease progression on surveillance biopsy.
Attempts at prevention of prostate cancer have had mixed results. While both dutasteride and finasteride reduced the incidence of prostate cancer in large, randomized clinical trials, both agents were associated with an increase in high-grade, potentially more aggressive, cancers. Notably, long-term follow-up studies have not shown an increase in prostate cancer mortality with these drugs. Nevertheless, neither has been approved by the Food and Drug Administration for cancer prevention. Unfortunately, thus far, studies of diet, vitamin supplements, and food agents have not revealed a clear path forward for primary prostate cancer prevention.
In some cancers, there is a clear precancerous stage that is being used as a target for prevention efforts, such as polyps in the colon and rectum. In the prostate, high-grade prostatic intraepithelial neoplasia, or HGPIN was hoped to be such a target. While HGPIN is often identified in biopsy specimens showing prostate cancer, it is relatively uncommon in prostate biopsies that do not show prostate cancer and it is not clear that the presence of HGPIN on a prostate biopsy increases a man’s risk of developing prostate cancer.
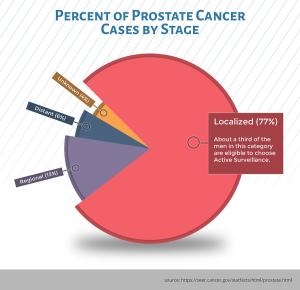
Prostate cancer in the United States is extremely common, making up nearly 10% of all cancers diagnosed (191,130 new cases are anticipated in 2020). Nearly 80% (see graphic) of these cancers are localized to the prostate gland, and a third are sufficiently low-risk that they may never require active treatment.
What kind of medical care are these men receiving after their prostate cancer diagnosis? While many men still choose to undergo definitive therapy (radical surgery or radiation) more and more men with low-risk disease are opting for “Active Surveillance” (see box). While this approach allows many men to avoid the complications associated with prostate cancer treatment, about a third of men who choose Active Surveillance are recommended to undergo surgery or radiation within 2 to 5 years due to evidence of disease progression. Therefore, men on Active Surveillance represent an ideal cohort in which to evaluate novel approaches to slow or prevent disease progression. A successful approach in this population might provide insight into preventing prostate cancer in men without the disease.
Research is under way in two key areas: vaccines and androgen blockers:
A vaccine developed at the National Cancer Institute, Prostvac™ (PSA-TRICOM), was designed to help the immune system to recognize prostate specific antigen (PSA) as a target and has been extensively studied in men with advanced prostate cancer. Although PSA-TRICOM did not increase the survival of men with metastatic prostate cancer that was not sensitive to treatment with hormones, it continues to be studied in earlier disease states in conjunction with other therapies.
Preventing the progression of localized disease is a much different outcome than attacking disease that has already spread through the body, so the hope is that augmenting the immune response might help men with early disease. A trial of PSA-TRICOM in 154 men receiving Active Surveillance for their prostate cancer is near completion. Men received 7 vaccine injections over 5 months, with measurement of the immune response in prostate tissue being the primary endpoint. The trial is expected to report out in June of 2020, and if a robust immune response is seen, the next step would be a larger trial with additional clinical endpoints.
Studies of next generation testosterone blockers are also under way in men on Active Surveillance. Apalutamide inhibits binding of androgens to the androgen receptor, turning off a signal for tumor growth, and the drug is approved for the treatment of more advanced disease. Two trials of this drug in the Active Surveillance population, one in the U.S. and one in France, are underway to help address concerns about potential side effects related to all androgen receptor blockers, such as erectile dysfunction and hot flushes.
A study of low-dose apalutamide is now in development by the NCI Cancer Prevention Clinical Trials Network (CPCTNet). In this study, researchers will assess the effect of decreasing doses of this drug on PSA and testosterone levels, seeking to balance the best preventive effects with the least side effects.
Selected References
- Goodman PJ, Tangen CM, Darke Ak, et al. Long-Term Effects of Finasteride on Prostate Cancer Mortality. N Engl J Med 2019 Jan 24;380(4): 393-394
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA: A Journal for Clinicians 2020 Jan 8;70(1): 7-30
- Gray PJ, Lin CC, Cooperberg MR, et al Temporal Trends and the Impact of Race, Insurance, and Socioeconomic Status in the Management of Localized Prostate Cancer. European Urology 2017 May;(71):729-737
- Klein EA, Thompson IM Jr, Tangen CM, et al Vitamin E and the Risk of Prostate Cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011 Oct 12;306(14):1549-56
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "Preventing the Progression of Low-Risk Localized Prostate Cancers: A New Frontier was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.
