Date Posted, by Alyssa M. Voss, M.P.H.
Researchers funded by NCI have developed and validated a new prostate cancer urinary biomarker test that can help differentiate who should proceed for biopsy after an elevated prostate-specific antigen (PSA) test from those who can wait.
The 18-gene test, called MyProstateScore version 2.0, or MPS2, improves upon the earlier version of MyProstateScore (MPS), the validated two-gene algorithm that uses urine prostate cancer antigen 3 (PCA3) and the TMPRSS2:ERG gene fusion to predict the presence of clinically significant prostate cancer among men with elevated serum PSA. Validation of MPS2 was completed through the NCI Early Detection Research Network (EDRN), and the results were published in JAMA Oncology.
Prostate cancer is the most commonly diagnosed cancer and the fifth leading cause of cancer death among men in the United States, disproportionately affecting African American men. A widely variable disease, prostate cancer can range from low-grade, slow-growing tumors that will never become life-threatening or require treatment, to high-grade, fast-growing cancers that can quickly spread to other parts of the body.

The most widely used prostate cancer screening methods are a PSA blood test and digital rectal exam (DRE), but these methods are imprecise. Though blood PSA levels will be elevated when cancer is present, PSA can also be high with non-cancerous conditions of the prostate, with low-grade, slow growing cancers, or if someone had a recent prior biopsy. Because of this imprecision, researchers have sought additional biomarker tests to use in conjunction with PSA to better identify which men may have a serious or more aggressive cancer that should be treated. As with any cancer screening approach, the goal is to reduce the number of cancer-related deaths.
“Clinicians need effective tools to reduce the harms of prostate cancer screening. One critical screening question that remains is, among men with an elevated PSA, who should have a biopsy”, said Howard Parnes, M.D., chief of the NCI Division of Cancer Prevention (DCP) Prostate and Urologic Cancer Research Group, who was not involved with the study. “The MPS2 has the potential to reduce unnecessary biopsies and is therefore an important step in the right direction.”
The new MPS2 urine test, developed by researchers at the University of Michigan, screens for 18 genetic markers that are highly associated with clinically significant prostate cancers – cancers that can grow quickly and should be monitored or treated – classified as grade group (GG) 2 and higher. The test is intended as an interim step before undergoing prostate biopsy among people who have an elevated PSA. For the test, men provide a urine sample at a clinic visit after DRE.
“This non-invasive test can serve as an added data point when discussing options with patients,” said senior author Arul Chinnaiyan, M.D., Ph.D., S.P. Hicks Endowed Professor of Pathology and Urology and director of the Michigan Center for Translational Pathology at the University of Michigan. “Approximately 75% of men who undergo biopsy do not have a cancer that needs to be treated. The MPS2 test provides additional information that can help avoid invasive biopsies and the discomfort and potential complications they bring.” A standard biopsy procedure involves systematically extracting 12 samples of prostate tissue from pre-specified areas of the gland.

To develop the MPS2 test, the team began by scanning a set of more than 58,000 potential genetic targets from pooled RNA sequencing data from the University of Michigan and the NCI’s The Cancer Genome Atlas. The panel was refined to a set of 54 candidate genes which, after running verification models, was whittled down to 17 genetic risk markers plus one reference gene, KLK3. The final MPS2 algorithm combines the 18 markers with other clinically relevant factors, such as age, race, and family history, to provide an overall result. Another version of the test, MPS2+, includes information about prostate volume in its algorithm.
The researchers then validated the assay using urine samples collected from 743 patients enrolled in an EDRN cohort of men who attended one of 11 academic sites for prostate biopsy. The samples went through MPS2 in addition to other clinically available prostate cancer biomarker tests for comparison. The results were then compared to patient biopsy results. They found that the MPS2 outperformed other validated prostate cancer biomarker tests at accurately identifying which patients had cancer detected at biopsy.
Further, the test showed a 95% sensitivity and negative predictive value (NPV) for GG2 cancers and 99% sensitivity and NPV for cancers GG3 and above. “This means that for someone who has a negative result, there is a very high confidence that no cancer is present,” said Chinnaiyan. The analysis estimates approximately 40% of unnecessary biopsies could be avoided using MPS2.
Putting the test to clinical use
So, what happens next? Chinnaiyan says now that the study results have been published, the assay will be submitted for consideration by the National Comprehensive Cancer Network, a group of 33 U.S. cancer centers that develops resources and guidelines to support high quality, equitable cancer care. He hopes the NCCN will include the MPS2 in their nationally recognized prostate cancer screening and clinical management guidelines, which currently endorses the original MPS and other validated biomarker tests.
But there is no need to wait - the test can be used by any clinician as long as a sample is sent to a CLIA-certified reference laboratory. The MPS2 assay has transferred to LynxDx, a diagnostic testing company Chinnaiyan founded to translate genomic biomarker tests into clinical practice. The company is working to have the assay reimbursed through Medicare and private insurance, but in the meantime, fees may be covered through an early access program. Ultimately the U.S. Food and Drug Administration will need to review and approve MPS2 if the test will be publicly marketed.
Important limitations of the validation study are also going to be addressed in ongoing research. Black men and men of races and ethnicities other than White have disproportionately high rates of prostate cancer mortality. Because the validation cohort included just 4% of its samples from African American men, additional studies are being done in EDRN cohorts that are more racially diverse to ensure the assay’s performance. Additionally, the assay will be validated in a population screened with multiparametric MRI, which provides a more informative three-dimensional image of the prostate and is becoming more frequently used as a second-line assessment after PSA.
The NCI Early Detection Research Network
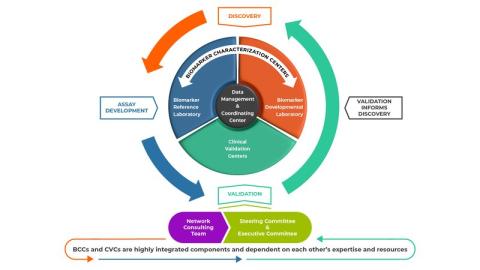
The Early Detection Research Network (EDRN) is a collaborative program that maintains comprehensive infrastructure and resources critical to the discovery, development and validation of biomarkers for cancer risk and early detection.
Administered through DCP’s Cancer Biomarkers Research Group (CBRG), EDRN connects multidisciplinary teams across the United States to fill gaps that had traditionally plagued the biomarker development process. Linking key players in the biomarker discovery, assay development, and validation areas allows seamless transition of candidate markers through the pipeline. The infrastructure also provides shared resources such as data and biologic samples, consultation and advisory services, and funding to ensure promising cancer biomarkers meet the highest standards for accuracy and quality through the network’s rigorous standardized testing protocols.
“The development of the MPS2 test provides an important, progressive improvement in current methods for the early detection of prostate cancer, said Sudir Srivastava, Ph.D., M.P.H, EDRN program director and chief of CBRG. “It takes a village of various expert teams to develop and validate an accurate cancer biomarker, and we consider this another EDRN success.”
To learn more about EDRN, visit https://prevention.cancer.gov/research-areas/networks-consortia-programs/edrn.
Reference: JJ Tosoian, Y Zhang, L Xiao, et al. Development and validation of an 18-gene urine test for high-grade prostate cancer. JAMA Oncology. 2024 doi:10.1001/jamaoncol.2024.0455
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "An Improved Prostate Cancer Biomarker Test May Help Men Avoid Unnecessary Biopsy was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.