Date Posted, by Jack J. Lee, Ph.D.
Tumor cells release telltale molecules into blood, urine, and other bodily fluids. But it can be difficult to detect tumor-derived DNA, RNA, and proteins in the earliest stages of disease, when cancers can be easier to treat and cure. Earlier stages shed fewer cancer cells—and fewer tumor markers.

Sudhir Srivastava, Ph.D., M.P.H.
“Endogenous markers may be there, but not detectable with current technology,” said Sudhir Srivastava, Ph.D., M.P.H., chief of the Cancer Biomarkers Research Group in the NCI Division of Cancer Prevention. The clinical detection of tumors is limited to masses larger than roughly one centimeter in diameter, which can reflect ten years of growth after the tumor first develops.
Rather than relying on endogenous signals, which originate within the body, some researchers are taking a new approach. They are using clever engineering technologies and tumor-specific biological information to trick a tumor to shed synthetic biomarkers that are detectable in biofluids when the tumor is still undetectable by any existing technology. The exogenously administered bioengineered sensors when delivered into the body, can produce a signal in the presence of cancer cells. Such methods have proven successful in mouse models and picked up much smaller tumor volumes.
“The merger of biology, engineering and the clinical field is very new and exciting,” said Sharmistha Ghosh-Janjigian, Ph.D., a program director for the Cancer Biomarkers Research Group.
Challenges in Early Detection
The adult human body contains about 5 liters of blood, on average. Detecting miniscule quantities of biomarkers diluted in this large volume presents a huge challenge. On top of that, the molecules do not simply stay in the blood. They degrade and are cleared by the kidney in minutes.
“It's like trying to fill a bathtub of water with the drain open,” said Gabe Kwong, Ph.D., associate professor of biomedical engineering at the Georgia Institute of Technology and Emory School of Medicine. “Your body is constantly clearing and cleaning the blood. Even if you have these biomarkers secreted into blood, they're cleared rapidly.”
Approaches that detect circulating tumor DNA can overcome such challenges through polymerase chain reaction (PCR). This method amplifies tiny numbers of DNA so they can be detected by scientific instruments. The same technology underlies COVID-19 tests, which pick up nucleic acid sequences from the SARS-CoV-2 virus. But there has not been an equivalent “PCR for proteins” to magnify protein levels.
Enzyme Activity
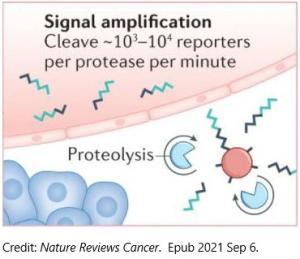
One solution to this detection challenge is to measure protein activity, rather than proteins themselves. Dr. Kwong and others have taken this approach to detect proteases, a type of enzyme that cuts up other proteins. Proteases play critical roles in the activity of normal cells, such as regulating signaling. But in cancer cells, they are unusually active and contribute to hallmarks of cancer, including invasion and metastasis.

Gabe Kwong, Ph.D.
Researchers have created synthetic biomarkers made up of inert carriers decorated with the protein targets of these proteases. When injected into mice, the assemblies circulate throughout the bloodstream. The protein targets are cleaved when they encounter tumor proteases. The pieces that have been cut off can then be detected in the blood or urine.
“We’re engineering protease sensors to amplify signs of early cancer,” Dr. Kwong said. The design also enabled the team to solve another challenge: proteases are promiscuous. The enzymes can cut a variety of different protein sequences, making it difficult to know with certainty whether signals were due to a tumor-associated protease. Fortunately, the engineers were not limited to just one protease or one target.
“It dawned on us that with engineered sensors, we are not limited by how many we use. Why not throw in 10 sensors simultaneously? Or 100?” Dr. Kwong said. Machine learning algorithms can then read these signals and produce sensitive, specific signatures for disease.
Dr. Kwong and colleagues first reported this approach in 2013, to monitor disease in mouse models of liver fibrosis and cancer. A system of 10 synthetic biomarkers detected tumors in mice when they were 60% smaller than tumors detectable by a clinically used blood biomarker for colorectal cancer.
More recently, Sangeeta Bhatia, M.D., Ph.D., who led the 2013 work, and colleagues at Massachusetts Institute of Technology described analogous activity-based nanosensors in a mouse model of lung cancer. These nanosensors could detect total tumor volumes that were only about 3 cubic millimeters on average.
Synthetic biomarkers may have applications beyond early detection as well. In a presentation at the 37th steering committee meeting of the Early Detection Research Network (EDRN), Dr. Kwong shared preliminary results describing activity-based biomarkers that provide an early readout for whether tumors respond to immune checkpoint blockade therapy.
Looking Forward

Sharmistha Ghosh-Janjigian, Ph.D.
Clinical trials evaluating activity-based synthetic biomarkers for disease detection in humans are still pending. A phase I study showed that a set of 16 biosensors was safe when injected in healthy human volunteers. While there are still challenges to overcome and questions to address, synthetic biomarkers have exciting promise for the early detection and prevention of cancer.
Drs. Bhatia, Ghosh-Janjigian, Kwong, and Srivastava co-authored an overview of the research field in the 20th anniversary issue of Nature Reviews Cancer, which looked back on 20 years of cancer research and forward to the future.
“We are very excited about this field and the tremendous promise and potential that it has,” Dr. Ghosh-Janjigian said.
References
Kirkpatrick JD, Warren AD, Soleimany AP, et al. Urinary detection of lung cancer in mice via noninvasive pulmonary protease profiling. Science Translational Medicine 2020; 12(537):eaaw0262.
Kwon EJ, Dudani JS, Bhatia SN. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nature Biomedical Engineering 2017; 1:0054.
Kwong GA, Ghosh S, Gamboa L, et al. Synthetic biomarkers: a twenty- first century path to early cancer detection. Nature Reviews Cancer 2021; 21(10):655-668.
Kwong GA, von Maltzahn G, Murugappan G, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nature Biotechnology 2013; 31(1):63-70.
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "Engineering Synthetic Biomarkers for Early Detection of Cancers was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.
