Date Posted, by Susan Jenks
Scientists have identified more than 1,000 potential new biomarkers for cancer that they hope will aid in the early detection of many of these complex diseases, including one of the most challenging, pancreatic cancer.
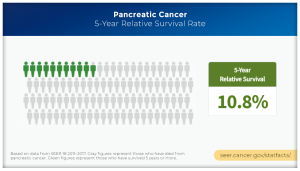
Pancreatic cancers have long defied early detection with the vast majority diagnosed in advanced stages when they are especially lethal. The 5-year relative survival rate for patients with widespread disease is just 3%. The 5-year survival for all stages in this cancer remains grim, less than 11%. If current trends persist, within a decade these relatively rare malignancies are projected to overtake colon cancer as the second leading cause of cancer death in the United States.
“Pancreatic cancer mortality rates are increasing and we don’t know why,” said Sudhir Srivastava, Ph.D., M.P.H., chief of the Cancer Biomarkers Research Group in the NCI Division of Cancer Prevention. “But, pancreatic cancers are considered curable if diagnosed in stage I and are amenable to resection,” he said.
Only a small percentage of patients, roughly 10% to 15%, fall into this potentially curable group. Difficulties arise in the early detection of this cancer, in part, because of its location deep within the abdomen: the cancer may cause no symptoms or symptoms are vague, mimicking other illnesses. Most early-stage pancreatic cancers are detected incidentally during imaging scans for other abdominal conditions.
In addition, no effective screening test yet exists for pancreatic cancer, which will be diagnosed this year in an estimated 60,430 men and women in the U.S. In 2019, an expert panel from the U.S. Preventive Services Task Force recommended against screening for pancreatic cancer in the general population, citing a high probability of overdiagnosis and medical interventions that pose more harm than benefit. As described in an earlier Cancer Prevention Science blog post, a new NCI-sponsored study is recruiting participants to evaluate different screening strategies for non-cancerous pancreatic cysts to determine which works best at detecting the few cysts that may become cancer.
A pancreatic cancer cell
Given this complicated scenario and the absence of other ways to find these cancers before they spread to regional lymph nodes or metastasize to distant sites, researchers have accelerated efforts to identify biomarkers that can be used to distinguish between normal biological processes in the pancreas and those that transform healthy cells into malignant ones. Investigators in the NCI’s Early Detection Research Network (EDRN) and Pancreatic Cancer Detection Consortium (PCDC) have identified many candidate biomarkers, using technologies such as next generation DNA sequencing and radiomics, a sophisticated imaging process that looks for aberrant patterns undetectable to the human eye.
Multiple Research Avenues Underway
By definition, biomarkers encompass a broad range of molecules from DNA and RNA to proteins and metabolites that signal biological changes as cancer develops, to exosomes, a grab bag of molecular constituents under study as a potentially rich source of biomarkers. Not only do cancer cells produce these diverse biological molecules, so do other cells and tissues in response to the presence of a growing cancer. Investigators are looking at multiple research avenues for biomarkers in tumors, blood, urine, and other bodily fluids.
In pancreatic cancer, CA 19-9 is considered the most extensively studied biomarker to date. A protein found on the surface of some but not all cancer cells, CA 19-9 is also expressed in several non-cancerous conditions, such as pancreatitis, or diabetes, which can muddy early detection results.
“For early detection, it’s not a good marker,” said Sharmistha Ghosh-Janjigian, Ph.D., program director for DCP’s Cancer Biomarkers Research Group. “And when you add in these co-morbid conditions, that’s where it gets tricky.” Although CA19-9 has proven useful in predicting whether a tumor is progressing, she said, its limited value in finding early disease has spurred research into other markers to use alone or in combination with CA-19-9. But even that has limits, as 10% of people with pancreatic cancer do not produce CA19-9.
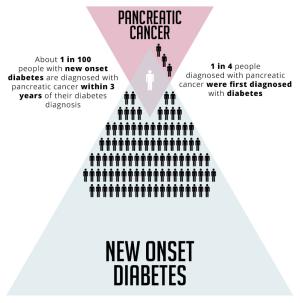
Pancreatic Cancer - New Onset Diabetes
Identifying a promising biomarker is just the beginning of a lengthy evaluation and review process that can take years, investigators said. Despite the explosion of biomarkers generated in cancer labs nationwide, the Food and Drug Administration has approved just eight for early cancer detection, risk assessment or support biopsy-related decisions.
Most ongoing research is still stuck in the earliest phases of development, according to Anirban Maitra, M.B.B.S., a pancreatic cancer expert who spoke at a scientific workshop of the NCI Early Detection Research Network. Each biomarker must first be benchmarked and then validated through “long and tortuous studies that need to be done,” said Dr. Maitra, a professor of pathology and translational molecular pathology at the University of Texas MD Anderson Cancer Center. Because a “utopian” biomarker does not yet exist, he said, it is far more practical to do surveillance studies, for now, in high risk populations: individuals with early-onset diabetes or familial histories, as well as those who test positive for any of 10 genetic mutations known to heighten individual risk.
Some hereditary markers carry a 13- to 39-fold increased risk, others a 53-fold increased risk, Dr. Maitra said, “but there’s a subset of patients where you could envision testing biomarkers.” Similarly, a small, but meaningful number of patients for testing might be found among individuals whose pancreatic cysts turn cancerous, he said.
As these efforts move forward, Dr. Maitra and his colleagues at MD Anderson are participating in a national research alliance assessing the comparative performance of a handful of biomarkers in early pancreatic disease. Described somewhat whimsically as a “biomarker bake-off,” the mission of teams at eight centers across the country is far from whimsical: to test a biomarker’s success at discriminating early-stage pancreatic cancer from benign disease and decide which ones merit further study.
Not limited to pancreatic cancer, the bake-off concept also applies to others, Dr. Srivastava said, amplifying the importance of biomarkers in moving all cancers closer to universal screening for multiple cancers. Although the idea of a single blood test remains a distant goal, the hope is that such a test could identify a broad spectrum of cancers long before symptoms appear. At least two biopharmaceutical companies are actively pursuing these multicancer tests.
NCI Director Ned Sharpless, M.D., has remarked on the potential value of multi-cancer early detection (MCED) tests to improve cancer screening—especially for cancers such as pancreatic cancer for which there are currently no screening modalities available—and the importance of rigorously evaluating these tests as they are developed.
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "The Search for Early Detection of Pancreatic Cancer Accelerates with Biomarkers was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.
