Date Posted, by DCP Staff
During chemotherapy for an advanced blood cancer, Stacey Blansky vaped marijuana daily for her nausea and used cannabis oil for anxiety and stress. The decision to add these complex, plant-derived substances to ease her treatment’s side effects came after doing her own research on possible benefits, she said.

In fact, while many patients say they experience symptom relief with cannabis products, there is no clear evidence on the benefits or potential harms, how cannabis interacts with the different cancer treatment agents, and whether it might modify or reduce the efficacy of treatments. At the same time, studying the efficacy and safety of cannabis continues to be challenging for researchers due to the patchwork of various restrictions in state and federal laws.
“Mine is a very ‘insider-outsider’ perspective,” Blansky told scientists at a recent four-day virtual symposium on Cannabis, Cannabinoids and Cancer Research, sponsored by the National Cancer Institute. “My oncologist wasn’t against my using cannabis, but she wasn’t well informed either. I had to push a bit to get it.”
Her physician’s reluctance to recommend cannabis illustrates one of the hurdles cancer patients face, even as state regulations have undergone what investigators described as a sea change in recent years.
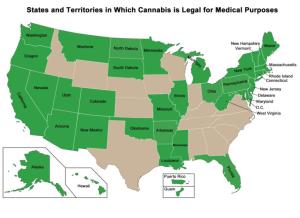
It remains illegal for most physicians to prescribe cannabis, including in states where cannabis is legal to use as a treatment.
Some 36 states allow medicinal use of these products while 15 states and the District of Columbia permit adult recreational or non-medical use. Three states prohibit the sale of non-FDA approved cannabinoids, the term for natural compounds derived from the cannabis plant, primarily THC (tetrahydrocannabinol) and CBD (cannabidiol).
THC and CBD share similar chemical structures, scientists said, but they differ in their biological effects as they interact with cannabinoid receptors in the brain that control pain, mood, sleep, and feelings. In general, researchers know more about THC than CBD, especially for symptom management of people with cancer and other diseases, such as HIV/AIDS and multiple sclerosis.
The shifting legal landscape has ushered in an explosion of diverse cannabis products in the marketplace and lent urgency to renewed public health efforts to assess their touted benefits and potential harms.
In some states, consumers now can buy everything from spiked gummy bears to an array of beauty products and cannabis-laced teas and tinctures. Many of these contain THC, CBD, or both, in unknown doses and with uncertain additives that enhance aroma and flavor. The use of the products varies as much as the products themselves, from straight-forward snacking of edible brownies and cakes to smoking pipes and bongs, or inhaling cannabis mixtures in vaping devices. More recently, a new delivery mode has aroused safety concerns, scientists said, called “dabbing” in which heated devices provide extremely high concentrations of THC.
Barriers to Obtaining Safety and Efficacy Data
“Why don’t we know more than we do?” asked Gillian Schauer, Ph.D., M.P.H., a research scientist at the Alcohol and Drug Abuse Institute of the University of Washington. “There are barriers, many of them,” she said, citing gaps in data monitoring and epidemiological studies on who uses these products and how; and overlap with use of other substances, such as alcohol and tobacco. While some clinical research has been done, most of those studies done, so far, have not been with the diverse cannabis products most readily available to consumers today.
Inconsistencies between state laws and a historic conflict with federal law further hampers scientific research, she and others said. Cannabis and cannabinoids fall into the illegal substances category under the federal Controlled Substances Act, except for industrial hemp-derived CBD, which was deregulated in 2018 and the two FDA-approved drugs containing modified forms of THC (dronabinol and nabilone). Defined as Schedule 1 drugs with a high potential for abuse and addiction, cannabis and cannabinoid products are regulated by the Drug Enforcement Agency, which restricts their access by researchers as well as by physicians outside of trials. Only physicians granted a special license may prescribe cannabis products, and those Schedule 1 licenses are rare.
Reflecting those constraints, the U.S. Food and Drug Administration has approved only two THC-derived drugs and one synthetic cannabinoid: Marinol® and SYNDROS™ (both made with dronabinol) are used to counteract the cachexia of cancer or AIDS and to mitigate nausea and vomiting from chemotherapy. Cesamet® (made with nabilone, a synthetic cannabinoid) is also used to reduce nausea and vomiting from chemotherapy. A fourth drug, Epidiolex™ (cannabidiol) is a cannabis-derived CBD product, that earned FDA approval in 2017 to ease seizures in two rare forms of epilepsy.
“We just don’t have the safety information we need, a weakness we’ve had for a long time,” Douglas Throckmorton, M.D., the deputy center director for regulatory programs in FDA’s Center for Drug Evaluation and Research, told workshop participants. “All the states are struggling with this issue and fundamentally, we need to do much better,” perhaps through setting up a national patient registry or collecting targeted data from patients’ real-world experiences, as to dose and duration of use, he said.
NCI is supporting researchers to carry out a survey of use in cancer patients. The survey includes tumor types, or which cancers provoke the highest cannabis use, and long-term follow-up. “This is the beginning of our mission to look at cannabis use among people with cancer,” said Gary Ellison, Ph.D., M.P.H., chief of the environmental epidemiology branch in the NCI Division of Cancer Control and Population Sciences. “The proliferation of laws out there necessitates the need to better assess the impact on health.”
Some scientists suggested that the strongest evidence supporting cannabis use may be for symptom management of chemotherapy-induced nausea and vomiting. Others noted there is very preliminary evidence that cannabis and cannabinoids may help patients sleep better, improve appetite and lessen neuropathic pain associated with HIV, diabetes, and chemotherapy-induced neuropathies. A few researchers want to focus on the possibility that cannabis has anti-cancer activity.
Ms. Blansky said she does not know if marijuana or cannabis oils helped put her Hodgkin’s lymphoma into remission over a year ago, although she feels their use helped her cope with her treatment. She described her goal in speaking before the symposium as “trying to change the narrative in some way,” to debunk myths about cannabis products as gateway drugs and to help to decriminalize their use in people with cancer.
Part 2 of this blog addresses gaps in research about cannabis and cancer.
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "Cannabis and Cancer, Part 1: Despite Lack of Evidence, Cannabis Products Being Widely Used was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.