
The Discovery and Development of Natural Products for Cancer Interception and Prevention Program (DDNP-CIP) supports the discovery and development of new natural products that are safe, non-toxic, and useful for cancer interception and prevention. Given the wide range of chemical diversity found in natural products around the world, they present an opportunity to discover biologically active compounds with unique structures and mechanisms of action. However, only a small percentage of them have been screened and evaluated for their potential in cancer prevention. NCI has one of the most diverse libraries of semi-purified natural product fractions in the world that are readily available to the research community for further testing. DDNP-CIP investigators are using new techniques, including high-throughput screening strategies, to screen natural products for activity in pathways to intercept and prevent cancer.
On This Page
- All Heading 2s will automatically be pulled in to this list.
- Do not edit the content on this template.
About DDNP-CIP
The Discovery and Development of Natural Products for Cancer Interception and Prevention (DDNP-CIP) Program’s overall research objectives are to:
- Identify and select clinically relevant cancer interception and prevention pathways and targets in natural products;
- Develop robust high-throughput screening strategies and specific cell-based and/or cell-free assays to screen non-toxic natural agents;
- Screen, purify, and identify the structure of active natural compounds;
- Develop models that could be used to guide the selection of preventive agents active in assays.
The flow chart below shows the steps for discovery and development of natural products for cancer prevention The National Cancer Institute supports the process across divisions and the NCI Program for Natural Product Discovery (NPNPD). In addition, the National Institutes of Health National Center for Advancing Translational Sciences (NCATS) supports this process.
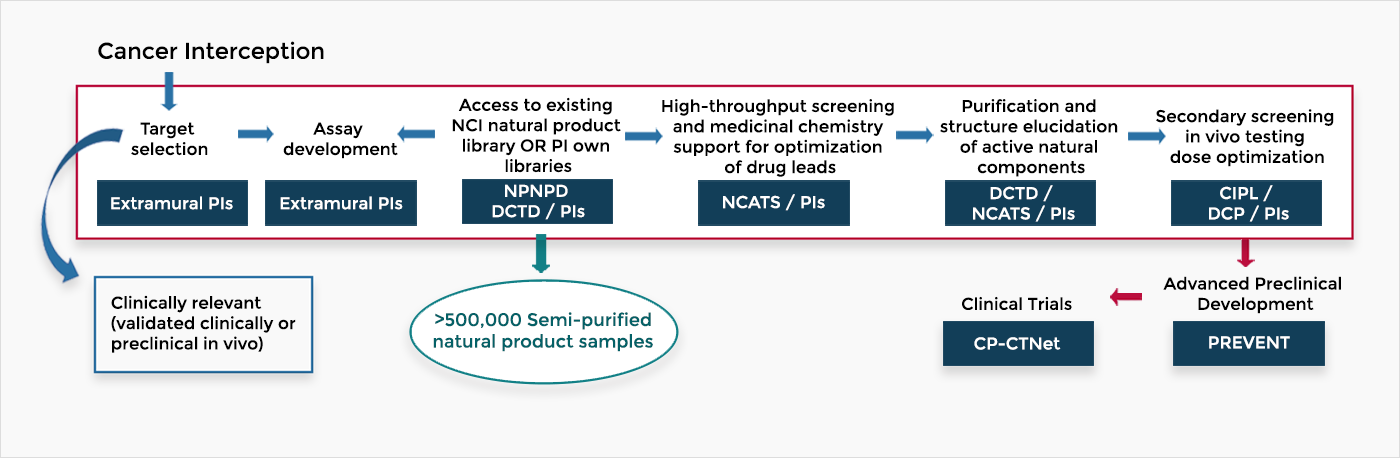
Investigators in the Discovery and Development of Natural Products for Cancer Interception and Prevention take advantage of NCI’s large library of “ready-to-screen,” pre-fractionated natural products to speed up bioassay-directed isolation and characterization of potential prevention agents. New natural agents discovered will move to the existing advanced preclinical development program, PREVENT, for further development towards early phase cancer prevention clinical trials by the Cancer Prevention Clinical Trials Network.
Funding Opportunities
No matching Funding Opportunities were found.
Program Contact(s)
Altaf Mohammed, Ph.D.
Email: altaf.mohammed@nih.gov
