Date Posted, by DCP Staff
As cancer researchers delve into the many molecular processes that guide cell life within the body, NCI is looking for ways to characterize and measure different substances in this process, with an eye to identifying who has cancer or who is at risk for the disease. One ongoing funding opportunity is promoting research on the isolation and characterization of extracellular vesicles (EVs) and their cargo for the discovery of biomarkers to predict cancer and cancer risk.
EVs are membrane-enclosed vesicles that are secreted by all cells. EVs are circulated in the blood and are readily accessible in most body fluids. The cargo of these vesicles includes RNA, DNA, proteins, metabolites, and lipids that reflect the cell of origin, making EVs appealing for biomarker development as part of the goal to create liquid biopsies.
In cancer, the cargo of the circulating EVs may mirror the altered molecular state (e.g., over- or under-expressed key proteins and microRNAs) of the cell of origin. Over the past decade, a number of studies have implicated EVs in major tumor-related pathways such as hypoxia-driven epithelial-to-mesenchymal transition, cancer stemness, angiogenesis, malignant formation, metastasis, and drug resistance. However, the findings have been inconsistent and variable.
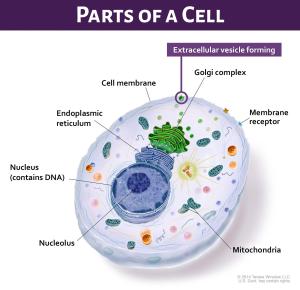
EVs are formed on the cell membrane to move their RNA, proteins, or other cargo into the environment outside the cell. Once outside the cell, these EVs can function as mediators of intercellular signaling, can modulate tissues, and can regulate inflammatory and immune responses. Cancer cells release more EVs than normal cells and EVs secreted from tumor cells can promote tumor progression, survival, invasion and angiogenesis. The analysis of exosomes and other EVs isolated from blood or other body fluids could provide insight into cancer cell biology.
EVs have potential for the development of non-invasive markers for early detection of various cancers due to their unique properties, including their stability in biological fluids and their potential to be efficiently isolated. Although work in several different laboratories centered on the analysis of EVs and their components isolated from normal and cancer cell lines, and isolation from biological fluids of cancer patients is ongoing, more research including case-control and prospective studies are needed to be conducted. In addition, EVs, as well as the molecules contained in EVs, may have the potential to be multiplexed–that is, incorporated into a single signal or test– with other molecular markers or screening modalities (e.g., imaging) to develop integrated molecular-based computational tools for the early detection of cancer.
EVs contain a diversity of membrane markers specific to the cells of origin on their outer surface, which can be used to distinguish cancer-derived EVs from EVs secreted by normal cells. EVs can also be isolated based on their size and density using a variety of methods.
The isolation of cancer-specific EVs could provide a source of biomarkers that are free of contaminating non-cancer EVs and other circulating contaminants. Purified EVs contain a molecular signature of the cancer cell of origin and the computational analysis of the RNA, DNA, proteins, metabolites, and lipids in theses vesicles may increase the sensitivity of the biomarkers for early detection, progression and metastasis.
One challenge is the lack of robust and reproducible methods for the isolation of a pure vesicular population. There is a lack of clear consensus for an optimal method of isolation of a pure EV population that is devoid of contamination with similar-sized vesicles of different origins.
Liquid biopsy, while offering great promise for early cancer detection, faces challenges to move the hype to reality. This is the reason for setting up the standards for technology, study designs, and methodologies which are so critical in ensuring reproducibility and rigor in the application of liquid biopsy in cancer detection and diagnosis. This initiative, the first of its kind, requires applicants to describe the proposed research plan to address the reproducibility issue by providing a clear roadmap to achieving their project objectives.
Through this funding opportunity, the Program to Assess the Rigor and Reproducibility of Extracellular Vesicle-Derived Analytes for Cancer Detection (R01 Clinical Trial Not Allowed, PAR-20-053), it is expected that successful applicants will critically evaluate and refine the existing methodologies and will agree to share their own methodological details to improve utilization of EVs in clinical use.
Sudhir Srivastava, Ph.D., M.D., and Mathew Young, Ph.D. of the Cancer Biomarkers Research Group in NCI’s Division of Cancer Prevention are the program directors for the PAR which begins accepting applications on January 5, 2020, using standard grant due dates through January 2023.
Previous versions of this funding opportunity (PAR-16-276 and PAR-16-277) resulted in several ongoing projects.
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "Taking Advantage of the Transportation Processes from Inside to Outside the Cell to Detect Cancer was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.