As the individual research teams working on their projects and joined U01 meetings, several ideas became cross-collaborations and led to the formation of working groups. The purpose of these are to delve into a specific idea and support the consortium and research community, at-large.
On This Page
- All Heading 2s will automatically be pulled in to this list.
- Do not edit the content on this template.
GP5
- Chair: Devin Peipert
- Members: Ethan Basch, Dave Cella, Eva Culakova, Amylou Dueck, Patricia Ganz, Bellinda King-Kallimanis, Sandra Mitchell, Devin Peipert, Jessica Roydhouse, Gita Thanarajasingam, Lynne Wagner, Fengmin Zhao
- Purpose: This goal of the GP5 working group is to confirm findings from the EVOLV team’s work around GP5 and to expand methodological applications of GP5. There is a particular focus on application in trials outside the ECOG ACRIN network.
Standardization Working Group
The Standardization Working Group of the US NCI Cancer Moonshot Tolerability Consortium was tasked with creating a resource to standardize analytic methods, visualization and reporting of tolerability data.
- Chairs: Lauren Rogak and Gillian Gresham
- Members: Mallorie Fiero, Erica Horodniceanu, Bellinda King-Kallimanis, Blake Langlais, Gina Mazza, Sandra Mitchell, Flora Mulkey, Luke Peppone
- Purpose: To identify, develop and communicate standardized approaches to the collection, analysis, graphical representation, and reporting of clinical trial tolerability data, including patient-reported outcomes.
An interdisciplinary group of members of the US NCI Cancer Moonshot Tolerability Consortium who meet on a monthly basis to discuss the development of a toolkit to be made available on the NCI website as a resource. The toolkit will include a suite of approaches to the selection and labeling of tolerability outcomes, standardization of tolerability metrics and analyses, and graphical representations, with the goal of providing a framework for the consistent communication and reporting of tolerability data, including PROs.
- Milestones:
- Creation of resource tables highlighting programming and statistical approaches
- Created guidelines for standardizing color schemes in graphics and other visual presentations
- Graphical representation of missing data in PRO-CTCAE
- Creation of resource tables highlighting programming and statistical approaches
Common Data Elements
Formed in 2019, the Common Data Elements working group was tasked with identifying common data elements present in studies from the four participating centers. Our overarching goal was to increase understanding of tolerability, with an operational goal that the identification of the common data elements would assist with sharing analytic procedures and approaches across consortium sites. Membership included representatives from the four grantees, the FDA, and the NCI.
- Chair: Marie Flannery
- Members: Working Group members: Marie Flannery (lead), Eva Culakova, Ginna Mazza, Fengmin Zhao, Greg Yothers, Andre Rogatko, Mallorie Fiero, Flora Mulkey, Sandra Mitchell, Diane St. Germain, Andrea Denicoff, Lori Minasian.
- Purpose: To create a typology that consisted of general categories related to understanding tolerability and then to specify specific data elements within each category. Representatives from each site then completed an excel spreadsheet, identifying available variables for each study on which data was already collected and available (as of Fall, 2019).
Summary of findings: The summary findings of the working group are being used by consortium members as a resource to examine across study commonalities (and absences) as one factor in determining shared approaches and analytic strategies. We learned both what data elements were commonly collected and also what elelments were not routinely present.
In total 21 studies were catalogued including 14 Phase III RCTs. Patient populations varied widely (e.g. breast cancer, melanoma, colon cancer, advanced cancer, and older adults with cancer). The common data elements identified are summarized in Table 1.Variables in 15 or more of 21 studies Demographic Age, sex, race, ethnicity Clinical Physician Rated Performance Status, Height, Weight Cancer Related Diagnosis, Stage, Current Treatment Tolberability Non-PROCTCAE CTCAE, Death, Treatment Discontinued, Dose Reduction, Hospilalization Tolerability Patient Reported PRO-CTCAE
Quality of LifeTable 1. Common data elements
Findings specific to PRO-CTCAE
A focus of the consortium is to evaluate patient reported tolerability outcomes, 15 of 21 studies included PRO-CTCAE items, they were collected at varying intervals (e.g. every cycle, every 3 months) and at 3-22 different time points. The number of PRO-CTCAE items ranged from 6-52, representing 3-46 different symptoms. The most frequently collected PRO-CTCAE are displayed in Figure 1.
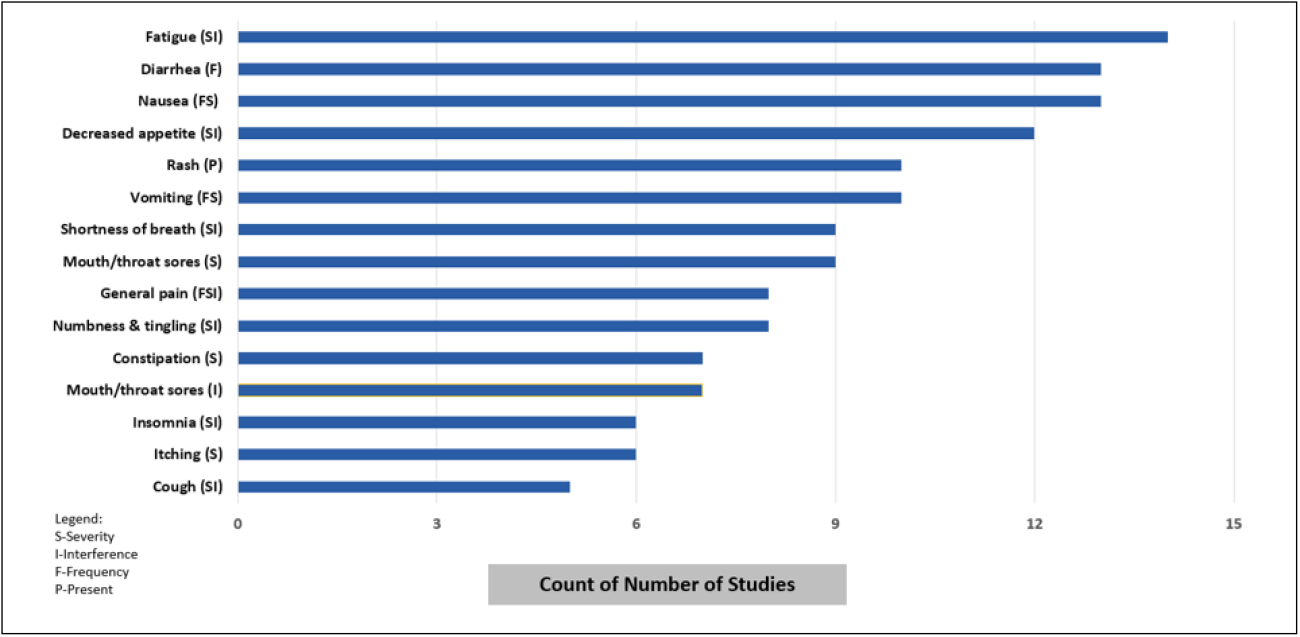
Figure 1. Most common PRO-CTCAE items identified from 21 studies
