The toxicity and tolerability of cancer therapies are important for many reasons: patients who experience bad side effects may not be able to finish their full course of treatment, reducing their chance of successful treatment. Often, side effects of cancer treatment may be bearable in the short term but have a significant effect on a patient’s quality of life that can last longer than the treatments.
For many decades, when patients with cancer participate in clinical trials of new therapies, the clinicians assessed and recorded the adverse events – usually toxic reactions to treatment. Cancer clinical trials researchers standardized how they report the unwanted side effects as the Common Terminology Criteria for Adverse Events (CTCAE). In recent years, however, patients have been completing thorough assessments of the side effects they encounter. These are Patient-Reported Outcomes (PRO) of CTCAE.
On This Page
- All Heading 2s will automatically be pulled in to this list.
- Do not edit the content on this template.
What is the Consortium?
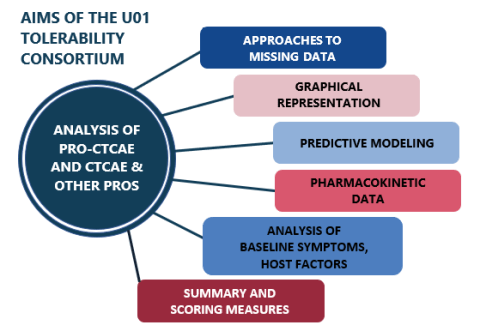
The purpose of this consortium has been to stimulate the development of methods for better understanding tolerability by analyzing the clinical trials adverse event data through the use of the Common Terminology Criteria for Adverse Events (CTCAE) and the Patient-Reported Outcomes version of the CTCAE (PRO-CTCAE™), as well as other clinically relevant data, e.g., stage of disease, laboratory findings, comorbidities, concurrent medications, including patient-reported outcomes (PROs) from cancer clinical trials.
The consortium is made up of four multidisciplinary funded research teams including principal investigators, biostatisticians, data scientists, investigators with patient-reported outcome (PRO) measurement expertise, cancer clinical trialists and patient advocates to develop and share analytic approaches to determine tolerability as well as develop a menu of methods for public use.
What is Tolerability?
Tolerability is currently defined as “the degree to which overt adverse effects can be tolerated by the subject” by the International Conference on Harmonization (ICH). A complete understanding of tolerability should include direct measurement from the patient on how they are feeling and functioning while on treatment. The current definition has necessitated the need for a more contemporary definition that better incorporates the patient experience using patient reported outcomes (PRO). Data elements and methodologies that provide a comprehensive characterization of tolerability are currently being explored to better inform patient and clinician treatment decisions.
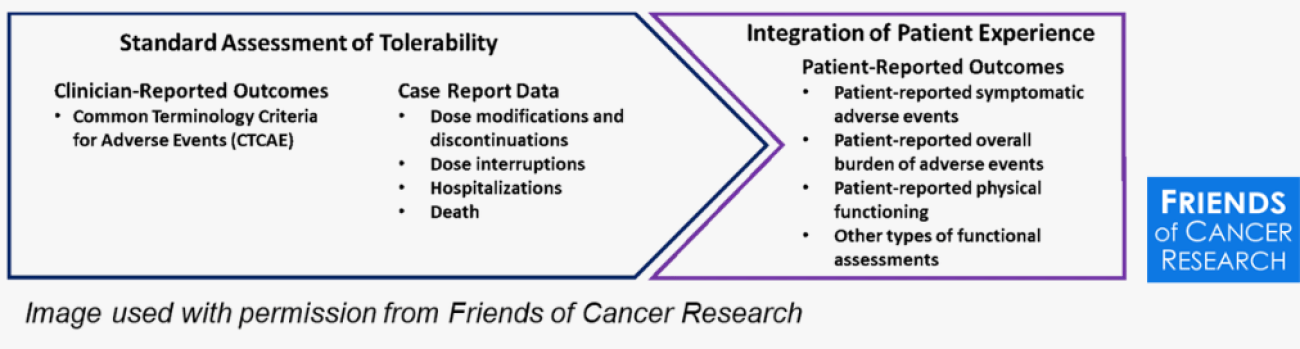
What is PRO-CTCAE?
The PRO-CTCAE item library includes 124 items representing 78 symptomatic adverse events and is a companion to the CTCAE. Each AE, up to three individual items, evaluate its frequency, severity, and interference with daily activities. PRO-CTCAE has been implemented in clinical trials across north america and internationally and is widely recognized as the standard to collect patient-reported symptomatic AEs to support treatment and patient care.
