Date Posted, by DCP Staff
As the availability and sophistication of smart technology for health (such as wearables, sensors, and micro-robotic tools) continues to increase, these devices could prove transformative in cancer prevention. Two ongoing Division of Cancer Prevention (DCP) funding opportunities are promoting the creation of smart devices to examine the interrelationships among nutrition, the gut microbiome, and human metabolism as tools for progress in cancer prevention research.
The Gut Microbiome's Important Role in Immunity
One DCP funding opportunity (PAR-20-133) seeks to examine the diet-host-microbiome interactions along the biogeography of the gastrointestinal (GI) tract using an ingestible micro-robotic pill. The other (PAR-20-134) focuses on the continuous monitoring of nutrients and their metabolites through wearable devices.
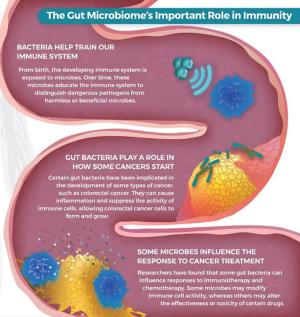
The gut microbiome plays an important role in the immune system’s ability to recognize and destroy cancer cells. From birth, the developing immune system is exposed to microbes. Over time, these microbes educate the immune system to distinguish dangerous pathogens from harmless or beneficial microbes. Researchers have found that certain bacteria in the gut microbiome can cause inflammation and suppress the activity of immune cells, allowing cancer cells to form and grow. In addition, some gut bacteria can influence responses to immunotherapy and chemotherapy. Some microbes may modify immune cell activity, while others may alter the effectiveness or toxicity of certain drugs. Evidence also suggests that chronic inflammation in the gut and associated mucosa can lead to the development of Inflammatory Bowel Disease-related cancers.
Gut microbiome explorers like smart pills or other ingestible smart devices will allow for monitoring and in situ interrogation and sampling of gut mucosal tissues along the various regions of the GI tract without disrupting normal gut physiology and affecting natural host-microbiota interactions. There are a lack of noninvasive devices and tools to study the real-time metabolic and molecular interactions of host-diet-microbiota in difficult to access GI regions. Smart pills might enable researchers to collect specimens of the GI mucosa or luminal content for a single time or repeated measures, while enabling the study of metabolic signaling in real-time. The devices could collect gut microbial samples from currently inaccessible sites along the GI tract without contamination and potentially provide the ability to culture them for interrogating specific diet-host-microbiota interactions.
Physiological biochemical sensors have already been developed for analysis of biochemical signals, such as blood glucose and electrolytes from body fluids (sweat, saliva, and tears). However, glucose is presently the only metabolite that can be monitored continuously for clinical and research purposes using smart technologies. Recent advances in skin-like biosensors (wearable devices) for noninvasive blood glucose monitoring via electrochemical channels have shown promise for the continuous monitoring of glucose. These devices open up new prospects to expand sensing capability to nutrients or metabolites beyond glucose, such as sodium, free calcium, choline, trimethylamine, amino acids, organic acids, microbiome metabolites, micronutrients, and phytonutrients. Additionally, multiplex or dual-purpose technologies, e.g., glucose/Na or glucose/insulin, might help in interpreting the measurements being made, for example, whether a result reflects changes in nutrient status or differences in the person, such as whether they are dehydrated.
Examples of tasks for ingestible smart devices includes the ability to:
- Monitor and create visualizations of in situ metabolic signaling and host-microbiota interactions along the GI tract;
- Perform minimally invasive laser capture microfluidic tools that allow single time or multiple sampling of gastric mucosa for reliable biochemical measurement;
- Enable delivery of dietary constituents to a single site or multiple locations in the GI tract in humans;
- Allow for interrogation of diet-microbiota-host interactions along discrete regions of the GI tract; and
- Enable collection of contaminant free microbial samples from distinct regions of the GI tract that can be cultivated in vitro to interrogate diet-microbiome interactions.
Examples of appropriate wearable devices include:
- Tools to monitor beta-hydroxy butyrate and other ketone bodies in circulation during a high fat, low carbohydrate diet;
- Sensors to measure circulating markers of inflammatory and immune function including cytokines in real time in response to dietary modification; and
- Sensors for continuous monitoring of nutritionally relevant small hormones or peptide hormones, such as insulin and glucagon, and other hormonal excursions during dietary intervention.
Through the funding opportunity, Gastrointestinal (GI) and Microbiome Explorers: Development of Swallowable Smart Pills or Devices for Precision Nutrition, Microbiome and Digestive Disease Applications (R21/R33 Clinical Trial Required, PAR-20-133), the goal is to yield implementable devices/tools for gastroenterological research or other clinical applications, along with monitoring and sampling of GI contents and/or mucosa to examine diet-host-microbiome interactions for clinical research or diagnostic applications. Roberto Flores, Ph.D., M.S. M.P.H., NCI DCP, and Padma Maruvada, Ph.D., National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), are the program directors for the PAR.
For the funding opportunity, Development of Wearable Smart Devices for Continuous Monitoring of Circulating Nutrients, Metabolites and Hormones (R21/R33 Clinical Trial Required, PAR-20-134, the goal is the creation of devices/tools that can continuously monitor a broader range of nutrients, metabolites and/or metabolic signals for advancing precision nutrition, microbiome, and circadian metabolism research. Sharon Ross, Ph.D., M.P.H., NCI DCP, and Padma Maruvada, Ph.D., NIDDK, are the program directors for the PAR.
Both funding opportunities have only two receipt dates: this past June, and June 2021. Applications already submitted are under review.
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. Please credit the National Cancer Institute as the source and link directly to the blog post using the original title, for example: "Smart Technology Is Aimed at Nutrition, Gut and Metabolism in Cancer Prevention Research Funding Opportunities was originally published by the National Cancer Institute." For questions, contact us at CancerPreventionBlog@mail.nih.gov.