Project Title/Research Areas: Multiplexed Multi-Modal Endoscopic Imaging of Cancer Biomarkers
Principal Investigator/Institution: Thomas D. Wang, M.D., Ph.D., University of Michigan, Ann Arbor, MI
Description of Current Activity
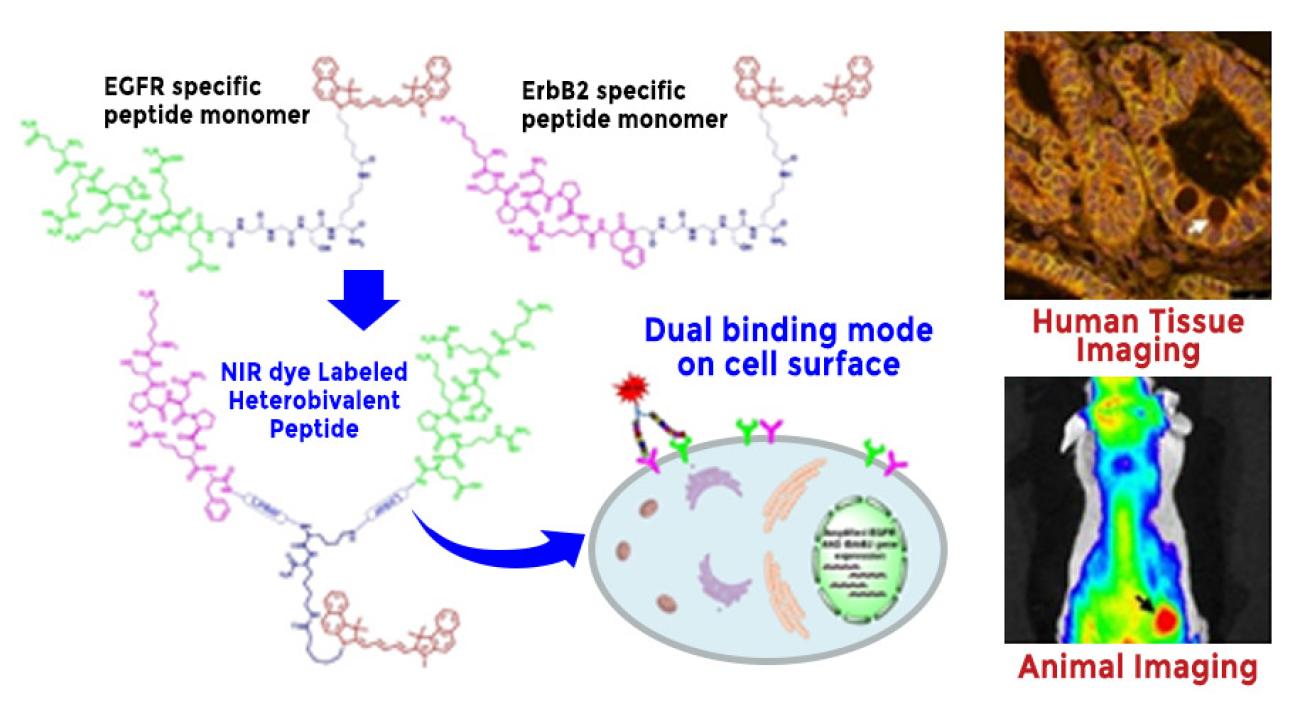
Esophageal adenocarcinoma (EAC) is a deadly cancer that is rising in incidence rapidly, and is associated with a poor prognosis and low 5-year survival. Barrett’s esophagus (BE) represents a metaplastic transformation of squamous epithelium, and is known to transform through low-grade dysplasia (LGD) and high-grade dysplasia (HGD). HGD is a pre-malignant condition that provides a window of opportunity for intervention with either curative resection or ablation therapy. Therefore, new methods for accurate detection of HGD and early EAC are needed. Multivalent ligands generate synergistic effects that can increase their affinity, avidity, selectivity, and potency by binding multiple targets concurrently. We aim to arrange heptapeptide monomers in a heterodimer configuration, and present the first fluorescently-labeled imaging agent that targets EGFR and ErbB2.
Update
We hypothesize that a heterobivalent peptide can be designed to target detection of early Barrett’s neoplasia by combining monomer heptapeptides specific for either EGFR or ErbB2 in a heterodimer configuration. We optimized this structure by identifying the length of a triethyleneglycol linker that maximizes binding interactions to these two surface receptors on OE33 human EAC cells. We demonstrated a Cy5.5-labeled heterodimer QRH*-KSP* that binds specifically binding to each target. We found 3-fold greater fluorescence intensity and 2-fold higher affinity compared with either monomer alone. Peak uptake in EAC xenograft tumors was observed at 2 hours post-injection with systemic clearance by about 24 hours in vivo. Furthermore, ligand binding was evaluated on human esophageal specimens ex vivo, and 88% sensitivity and 87% specificity were found for detection of either high-grade dysplasia (HGD) or EAC. This peptide heterodimer shows promise for targeted detection of early Barrett’s neoplasia in clinical study.
In the future, this dual targeting agent will undergo rigorous pharmacology/toxicology studies and will be synthesized under Good Manufacturing Practice (GMP) conditions for FDA approval to be used in human clinical studies with fluorescence endoscopy. This combination approach greatly simplifies the process for FDA review by comparison with that for two ligands separately. Development of bivalent peptides can be performed at a substantially lower cost than that for other protein-based agents separately to be used in combination therapy. Established peptide synthesis processes are easy to scale up for mass manufacture with reproducible results. By recognizing two different cell surface targets that are validated EAC biomarkers, enhanced diagnostic and therapeutic effects can be achieved as compared with ligands that bind only one target.