Project Title/Research Areas: Multiplexed Imaging of Biliary Intra-Epithelial Neoplasia
Principal Investigator/Institution: Eric J. Seibel, Ph.D., University of Washington, Seattle/ Thomas D. Wang, M.D., Ph.D., University of Michigan, Ann Arbor
Description of Current Activity
Cholangiocarcinoma is the most common malignancy of the biliary tract, and is increasing in incidence and mortality worldwide. The risk of this biliary tract cancer is increased greatly in patients diagnosed with primary sclerosing cholangitis (PSC), a chronic inflammatory disease characterized by inflammation, strictures, and scarring within the biliary ducts. Biliary tract cancer is usually diagnosed at an advanced stage, and results in poor overall prognosis. Relative survival rates are <5% when the cancer is no longer regionally localized. Patients who are diagnosed at an early stage are eligible for surgical resection and can have excellent outcomes; therefore, early detection is critical for long-term survival. This deadly disease usually presents on imaging as biliary strictures. However, strictures are a non-specific clinical finding and create an important diagnostic dilemma because they often result from benign conditions. Currently, there are no reliable methods for distinguishing indeterminate biliary strictures as progressing to cancer. No blood tests are diagnostic for cholangiocarcinoma and conventional endoscopic imaging with biopsy are low in both sensitivity and specificity. Accurate diagnosis is thus critical to avoid unnecessary surgery.
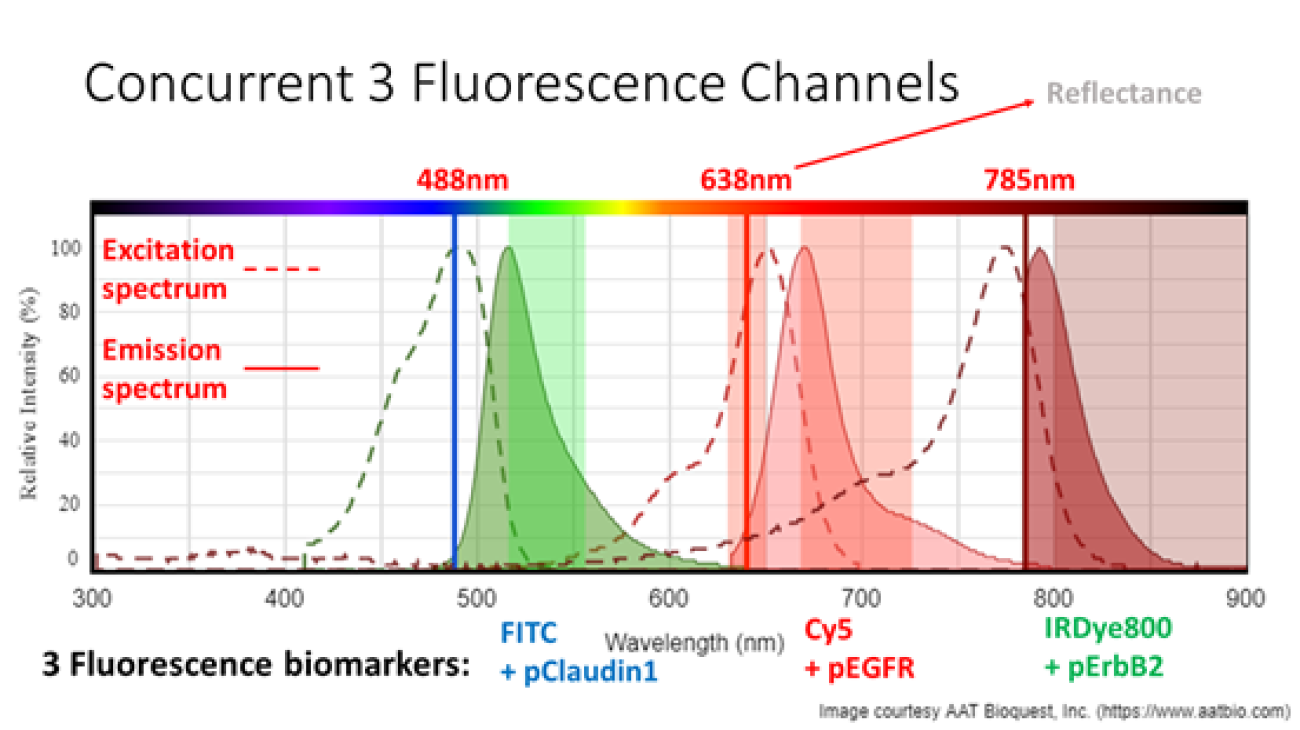
Molecular imaging is an emerging methodology for real time in vivo visualization of over-expressed targets that are highly specific for disease. To increase sensitivity, we are developing a multiplexed approach, imaging up to three different fluorescence peptide probes that are sensitive to early cholangiocarcinoma, called biliary intraepithelial neoplasia (BilIN). Confirmed from ex vivo tissue immunohistochemistry, the University of Michigan has selected two near-infrared fluorescence probes for BilIN: Cy5+pEGFR and IRDye800+pErbB2; and now approved for human clinical trials with a third still under development FITC+Claudin-1 (see Figure 1).
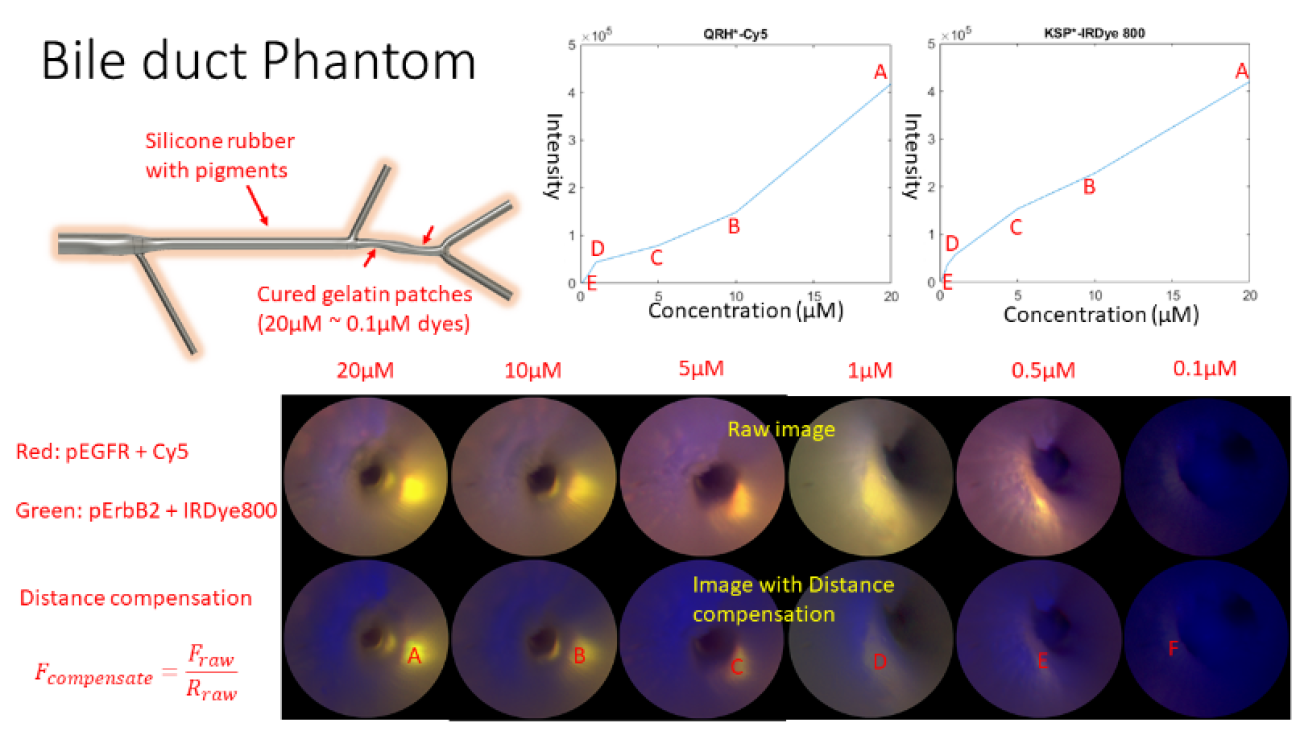
To clinically image BilIN, a custom ultrathin (1.2-mm diameter) and flexible endoscope was developed and tested at the University of Washington (UW) using multimodal imaging of bile duct phantoms, see Figure 2. Also, at the UW, a first-in-human clinical trial has been approved for the minimally-invasive endoscopic retrograde cholangio pancreatography (ERCP) procedure. The study goal is to produce quantitative fluorescence images of these peptide molecular probes within biliary structures, which are suspected of being/becoming cancerous. Future developments are the introduction of custom biopsy tools that are guided with simultaneous fluorescence and reflectance endoscopy to confirm the in vivo clinical images.
Broader impacts of this research are the development of molecular-based imaging tools in combination with other procedures (such as biopsy) to improve rates of early diagnosis and treatment of cancers in the small lumens of the human body, such biliary & pancreatic duct, fallopian tubes, and peripheral airways; as well as developing new interventional procedures in the cardiovascular system.